Effect of Neem (Azadirachta indica) on the Survival of Escherichia coli O157:H7 in Dairy Manure
Abstract
:1. Introduction
2. Experimental Section
2.1. Neem Materials and Extracts
2.2. Influence of Neem Materials on the Survival of EcO157 in Dairy Manure
2.3. Growth of EcO157 with Neem Extracts
3. Results
3.1. Survival of EcO157 in Dairy Manure Supplemented with Neem Materials

3.2. Growth of EcO157 with Neem Extracts
| Extract | Leaf Equivalents/Well a, mg | Concentration/Well, % |
|---|---|---|
| Aqueous | 12.5 | 4.2 |
| Ethyl acetate | 20 | 6.7 |
| Bicarbonate washed ethyl acetate | 20 | 6.7 |
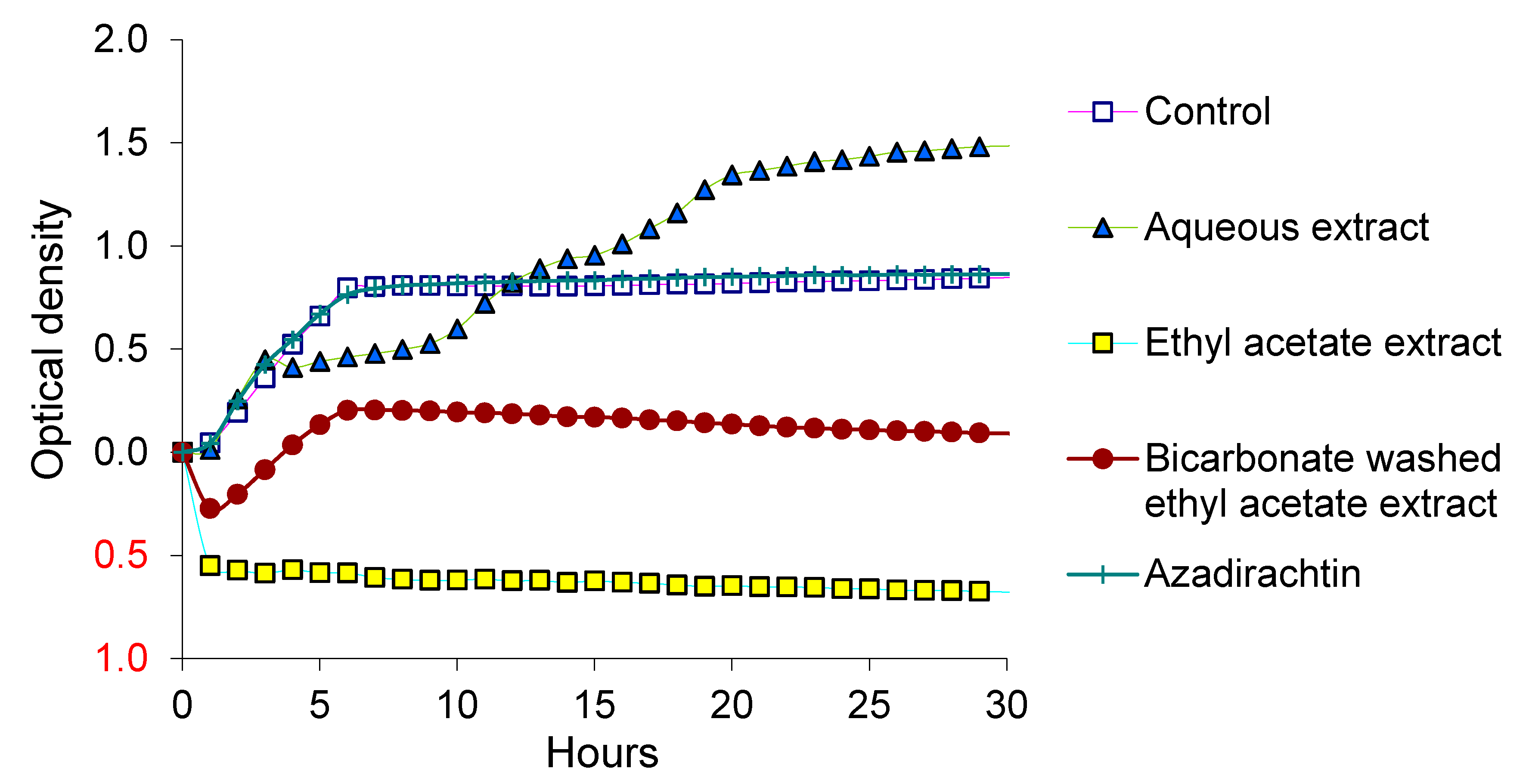
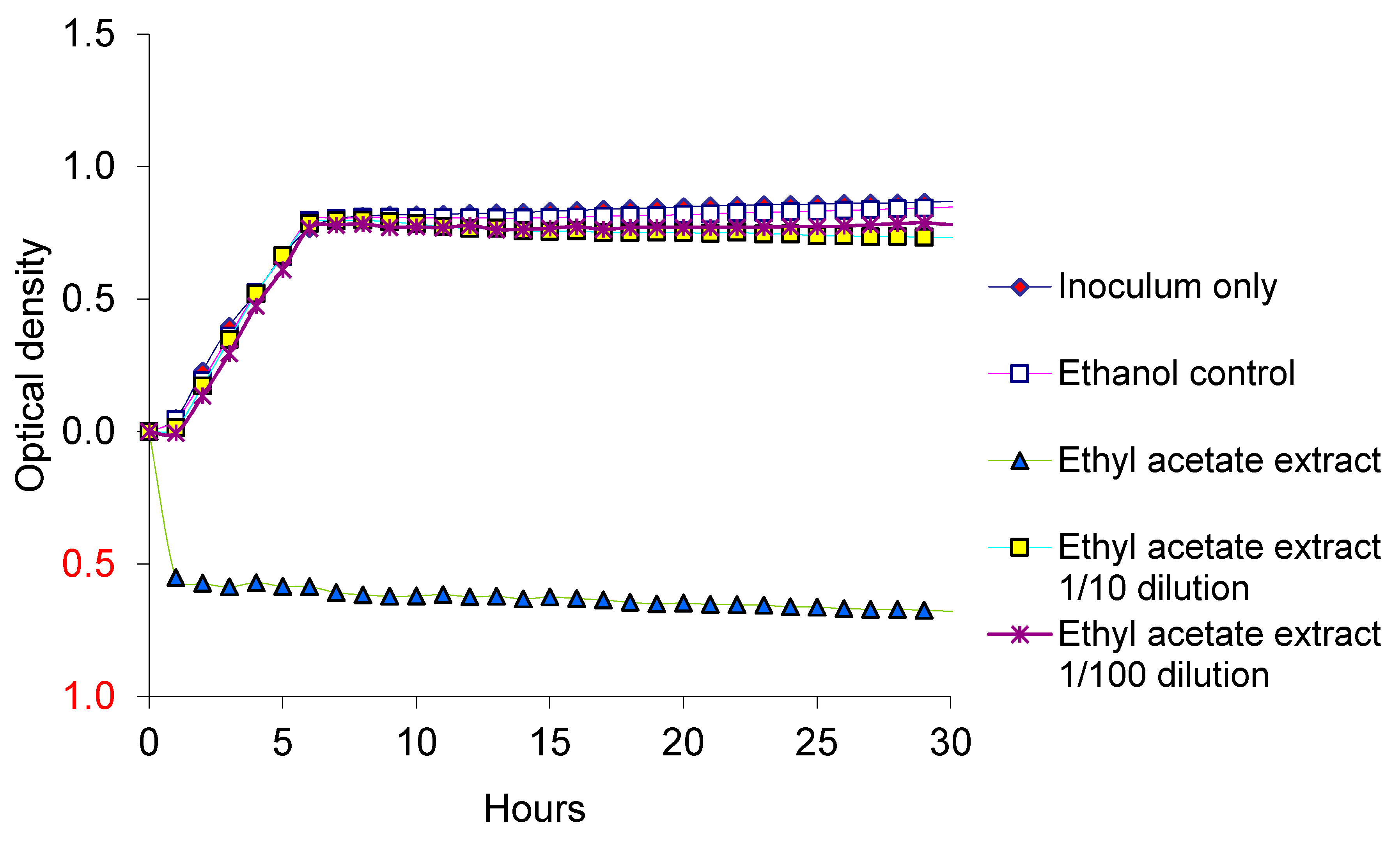
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cole, D.J.; Hill, V.R.; Humenik, F.J.; Sobsey, M.D. Health, safety, and environmental concerns of farm animal waste. Occup. Med. 1999, 14, 423–448. [Google Scholar] [PubMed]
- McGarvey, J.A.; Miller, W.G.; Sanchez, S.; Stanker, L. Identification of bacterial populations in dairy wastewaters by use of 16S rRNA gene sequences and other genetic markers. Appl. Environ. Microbiol. 2004, 70, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Elder, R.O.; Keen, J.E.; Siragusa, G.R.; Barkocy-Gallagher, G.A.; Koohmaraie, M.; Laegreid, W.W. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 2000, 97, 2999–3003. [Google Scholar] [CrossRef] [PubMed]
- Pell, A.N. Manure and microbes: Public and animal health problem? J. Dairy Sci. 1997, 80, 2673–2681. [Google Scholar] [CrossRef]
- Rasmussen, M.A.; Casey, T.A. Environmental and food safety aspects of Escherichia coli O157:H7 infections in cattle. Crit. Rev. Microbiol. 2001, 27, 57–73. [Google Scholar] [CrossRef] [PubMed]
- DeWaal, C.S.; Tian, X.A.; Plunkett, D. Outbreak Alert! 2009. Center for Science in the Public Interest. Available online: http://cspinet.org/new/pdf/outbreakalertreport09.pdf (accessed on 3 March 2015).
- Lynch, M.F.; Tauxe, R.V.; Hedberg, C.W. The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiol. Infect. 2009, 137, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Kanny, K.; Varaprasad, K.; Kumar, N.M.; Reddy, G.S. Novel-porous-Ag(0) nanocomposite hydrogels via green process for advanced antibacterial applications. J. Biomed. Mater. Res. A 2014, 102, 4616–4624. [Google Scholar] [PubMed]
- National Research Council. Neem: A Tree for Solving Global Problems; National Academy Press: Washington, DC, USA, 1992. [Google Scholar]
- Rao, D.S.; Penmatsa, T.; Kumar, A.K.; Reddy, M.N.; Gautam, N.S.; Gautam, N.R. Antibacterial activity of aqueous extracts of Indian chewing sticks on dental plaque: An in vitro study. J. Pharm. Bioallied Sci. 2014, 6, S140–S145. [Google Scholar] [PubMed]
- Jenner, F.; Jaleel, V.A.; Kulshrestha, R.; Maheswar, G.; Rao, P.K.; Kranthi, J. Evaluating the antimicrobial activity of commercially available herbal toothpastes on microorganisms associated with diabetes mellitus. J. Contemp. Dent. Pract. 2013, 14, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Chava, V.R.; Manjunath, S.M.; Rajanikanth, A.V.; Sridevi, N. The efficacy of neem extract on four microorganisms responsible for causing dental caries viz Streptococcus mutans, Streptococcus salivarius, Streptococcus mitis and Streptococcus sanguis: An in vitro study. J. Contemp. Dent. Pract. 2012, 13, 769–772. [Google Scholar] [PubMed]
- Bharitkar, Y.P.; Bathini, S.; Ojha, D.; Ghosh, S.; Mukherjee, H.; Kuotsu, K.; Chattopadhyay, D.; Mondal, N.B. Antibacterial and antiviral evaluation of sulfonoquinovosyldiacylglyceride: A glycolipid isolated from Azadirachta indica leaves. Lett. Appl. Microbiol. 2014, 58, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Thakurta, P.; Bhowmik, P.; Mukherjee, S.; Hajra, T.K.; Patra, A.; Bag, P.K. Antibacterial, antisecretory and antihemorrhagic activity of Azadirachta indica used to treat cholera and diarrhea in India. J. Ethnopharmacol. 2007, 111, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.X.; Cao, M.; Shi, D.X.; Yin, Z.Q.; Jia, R.Y.; Xu, J.; Wang, C.; Lv, C.; Liang, X.X.; He, C.L.; et al. Toxicological evaluation of neem (Azadirachta indica) oil: Acute and subacute toxicity. Environ. Toxicol. Pharmacol. 2013, 35, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Subapriya, R.; Nagini, S. Medicinal properties of neem leaves: A review. Curr. Med. Chem. Anticancer Agents 2005, 5, 149–146. [Google Scholar] [CrossRef] [PubMed]
- Mahfuzul Hoque, M.D.; Bari, M.L.; Inatsu, Y.; Juneja, V.K.; Kawamoto, S. Antibacterial activity of guava (Psidium guajava L.) and neem (Azadirachta indica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodborne Pathog. Dis. 2007, 4, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Mukherjee, S.C.; Sahu, B.B.; Murjani, G. Neem (Azadirachta indica) extract as an antibacterial agent against fish pathogenic bacteria. Indian J. Exp. Biol. 1999, 37, 1097–1100. [Google Scholar] [PubMed]
- Del Serrone, P.; Nicoletti, M. Antimicrobial activity of a neem cake extract in a broth model meat system. Int. J. Environ. Res. Public Health 2013, 10, 3282–3295. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.K.; Hina, M.; Tahir, M.M. Effect of Azadirachta indica (neem), sodium thiosulphate and calcium chloride on changes in nitrogen transformations and inhibition of nitrification in soil incubated under laboratory conditions. Chemosphere 2011, 82, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Ravva, S.V.; Korn, A. Extractable organic components and nutrients in wastewater from dairy lagoons influence the growth and survival of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Ravva, S.V.; Sarreal, C.Z.; Duffy, B.; Stanker, L.H. Survival of Escherichia coli O157:H7 in wastewater from dairy lagoons. J. Appl. Microbiol. 2006, 101, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Ravva, S.V.; Sarreal, C.Z.; Mandrell, R.E. Altered protozoan and bacterial communities and survival of Escherichia coli O157:H7 in monensin-treated wastewater from a dairy lagoon. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Ravva, S.V.; Sarreal, C.Z.; Mandrell, R.E. Identification of protozoa in dairy lagoon wastewater that consume Escherichia coli O157:H7 preferentially. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Mandrell, R.E. Enteric human pathogens associated with fresh produce: Sources, transport and ecology. In Microbial Safety of Fresh Produce: Challenges, Perspectives and Strategies; Fan, X., Niemira, B.A., Doona, C.J., Feeherry, F., Gravani, R.B., Eds.; IFT/Blackwell Publishing: Oxford, UK, 2009; pp. 3–42. [Google Scholar]
- Ravva, S.V.; Cooley, M.B.; Sarreal, C.Z.; Mandrell, R.E. Fitness of outbreak and environmental strains of Escherichia coli O157:H7 in aerosolizable soil and association of clonal variation in stress gene regulation. Pathogens 2014, 3, 528–548. [Google Scholar] [CrossRef] [PubMed]
- Kudva, I.T.; Blanch, K.; Hovde, C.J. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 1998, 64, 3166–3174. [Google Scholar] [PubMed]
- Jain, D.; Jayaram, L.; Prabhu, V.M.; Bhat, G.K. Antibacterial effect of neem (Azadirachta indica) oil on multidrug resistant bacteria isolated from human infections. Int. J. Biol. Med. Res. 2013, 4, 3544–3546. [Google Scholar]
- Dahiya, P.; Purkayastha, S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J. Pharm. Sci. 2012, 74, 443–450. [Google Scholar] [PubMed]
- Berry, E.D.; Wells, J.E.; Bono, J.L.; Woodbury, B.L.; Kalchayanand, N.; Norman, K.N.; Suslow, T.V.; Lopez-Velasco, G.; Millner, P.D. Effect of proximity to a cattle feedlot on Escherichia coli O157:H7 contamination of leafy greens and evaluation of the potential for airborne transmission. Appl. Environ. Microbiol. 2015, 81, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravva, S.V.; Korn, A. Effect of Neem (Azadirachta indica) on the Survival of Escherichia coli O157:H7 in Dairy Manure. Int. J. Environ. Res. Public Health 2015, 12, 7794-7803. https://doi.org/10.3390/ijerph120707794
Ravva SV, Korn A. Effect of Neem (Azadirachta indica) on the Survival of Escherichia coli O157:H7 in Dairy Manure. International Journal of Environmental Research and Public Health. 2015; 12(7):7794-7803. https://doi.org/10.3390/ijerph120707794
Chicago/Turabian StyleRavva, Subbarao V., and Anna Korn. 2015. "Effect of Neem (Azadirachta indica) on the Survival of Escherichia coli O157:H7 in Dairy Manure" International Journal of Environmental Research and Public Health 12, no. 7: 7794-7803. https://doi.org/10.3390/ijerph120707794





