A Method for the Preparation of Chicken Liver Pâté that Reliably Destroys Campylobacters
Abstract
:1. Introduction
2. Experimental Section
2.1. Identification of Chicken Liver Pâté Recipes and PROMPT Analyses
2.2. Liver Sources
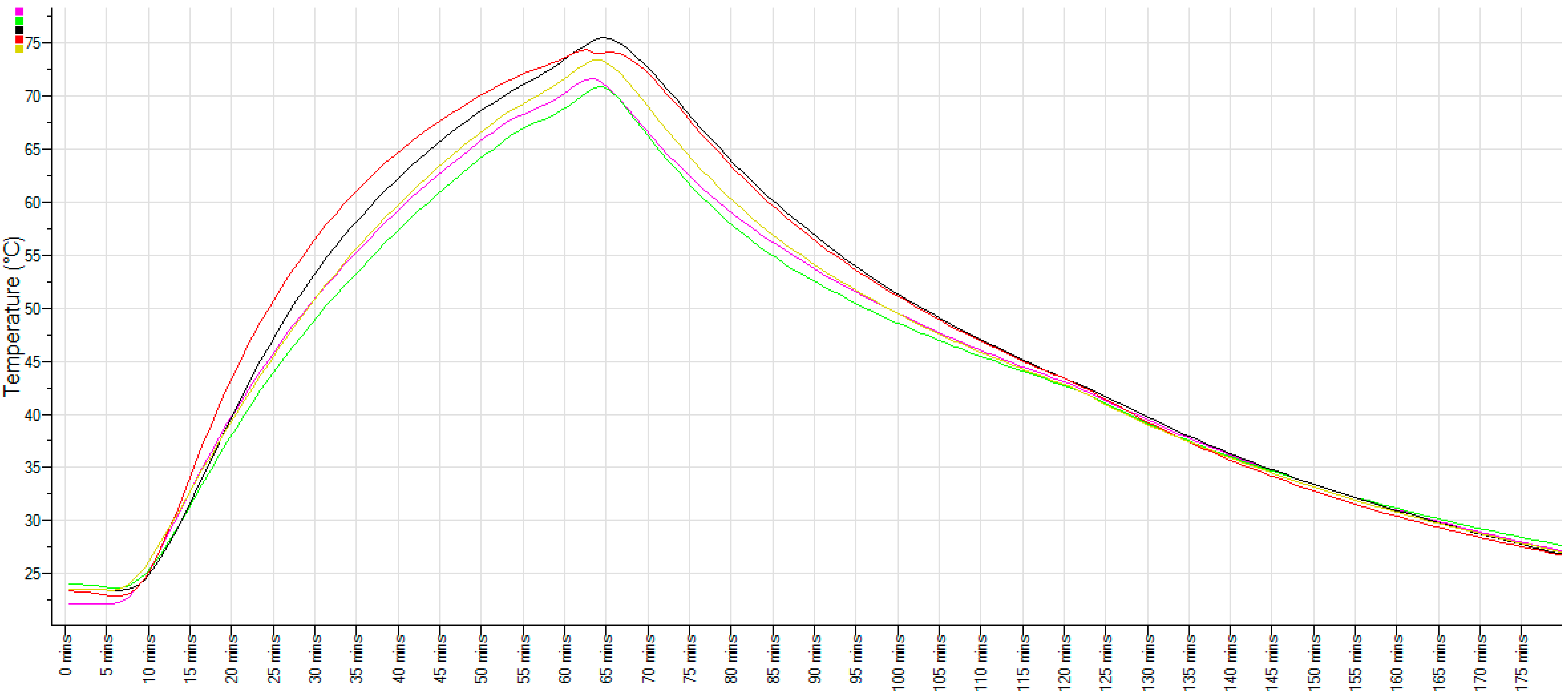
2.3. Temperature Monitoring of Cooked Livers and Bain Marie Cooked Pâté
2.4. Liver Washing
2.5. Microbiological Examination
2.6. Reflective Spectrophotometry
2.7. Sensory Analyses
| Attribute | Total Assessment Score for Pâté Made From | p Value | SEM a | |
|---|---|---|---|---|
| Fresh Livers | Frozen Livers | |||
| Color on surface | 70.5 | 67.1 | 0.29 | 3.25 |
| Color inside | 28.7 | 47.6 | <0.001 | 3.20 |
| Color likeability | 38.6 | 40.4 | 0.63 | 3.62 |
| Texture on cutting | 29.9 | 30.9 | 0.8 | 3.63 |
| On-eating firmness | 41.3 | 22.1 | <0.001 | 4.43 |
| On-eating dissolvability | 73.9 | 75.1 | 0.72 | 3.31 |
| Flavor strength | 52.7 | 61.9 | <0.001 | 2.21 |
| Fatty | 39.0 | 37.6 | 0.62 | 2.65 |
| Livery | 40.4 | 41.0 | 0.84 | 2.68 |
| Acidic | 8.4 | 12.8 | <0.05 | 1.93 |
| Rancid | 3.6 | 4.2 | 0.45 | 0.69 |
| Sweet | 30.6 | 30.7 | 0.94 | 2.33 |
| Peppery | 21.3 | 22.3 | 0.7 | 2.63 |
| Herby | 29.9 | 33.1 | 0.2 | 2.55 |
| Salty | 22.2 | 22.8 | 0.71 | 1.49 |
| Residue after eating | 20.1 | 7.8 | <0.001 | 2.84 |
| Flavor likeability | 44.8 | 51.0 | <0.05 | 2.65 |
| Overall likeability | 36.0 | 48.6 | <0.001 | 3.05 |
2.8. Statistical Analyses
3. Results
3.1. Compilation of a Pâté Manufacture Protocol
3.2. Thermal Processing
3.3. Effect of Washing
3.4. Preparation of Chicken Liver Pâté
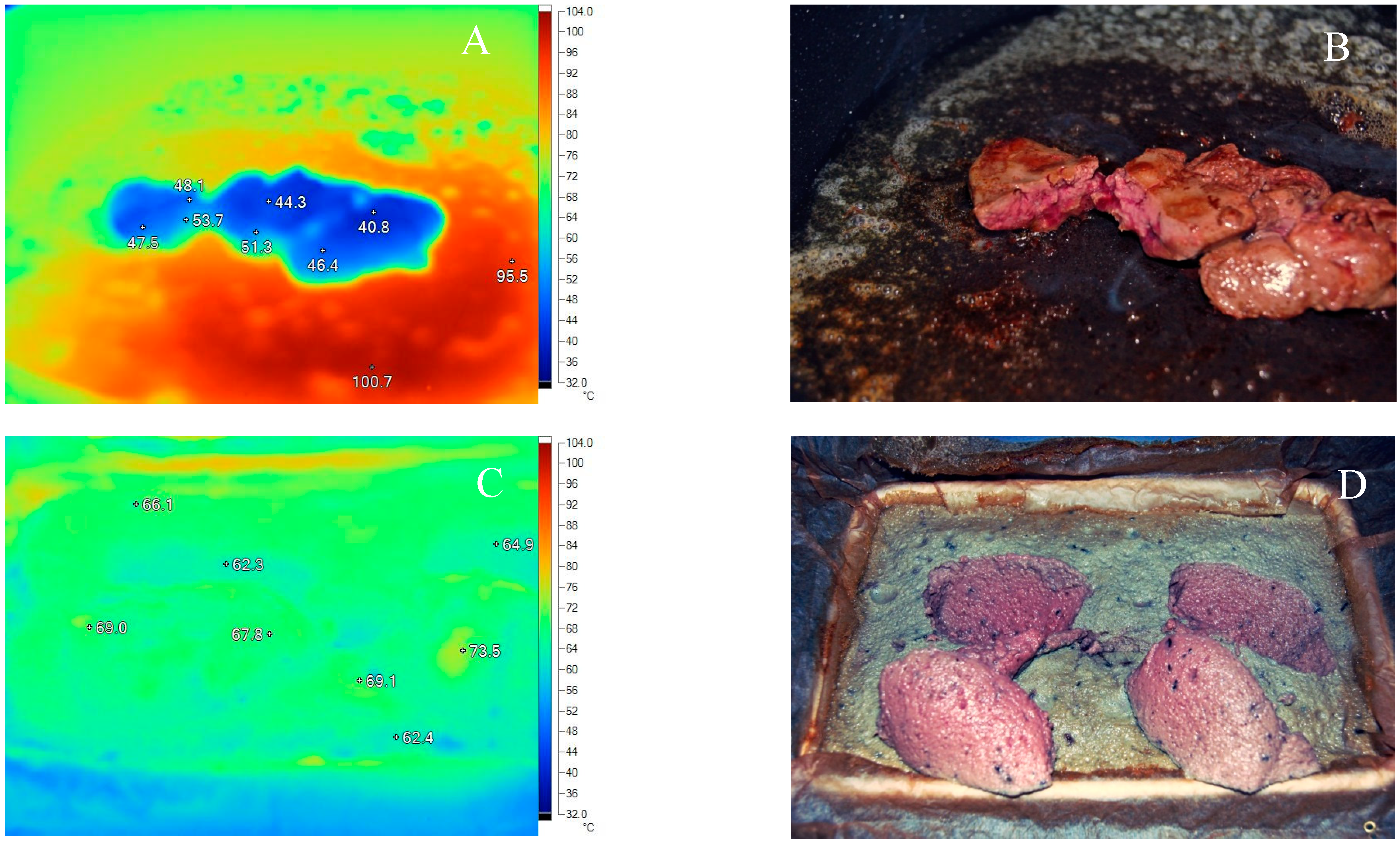

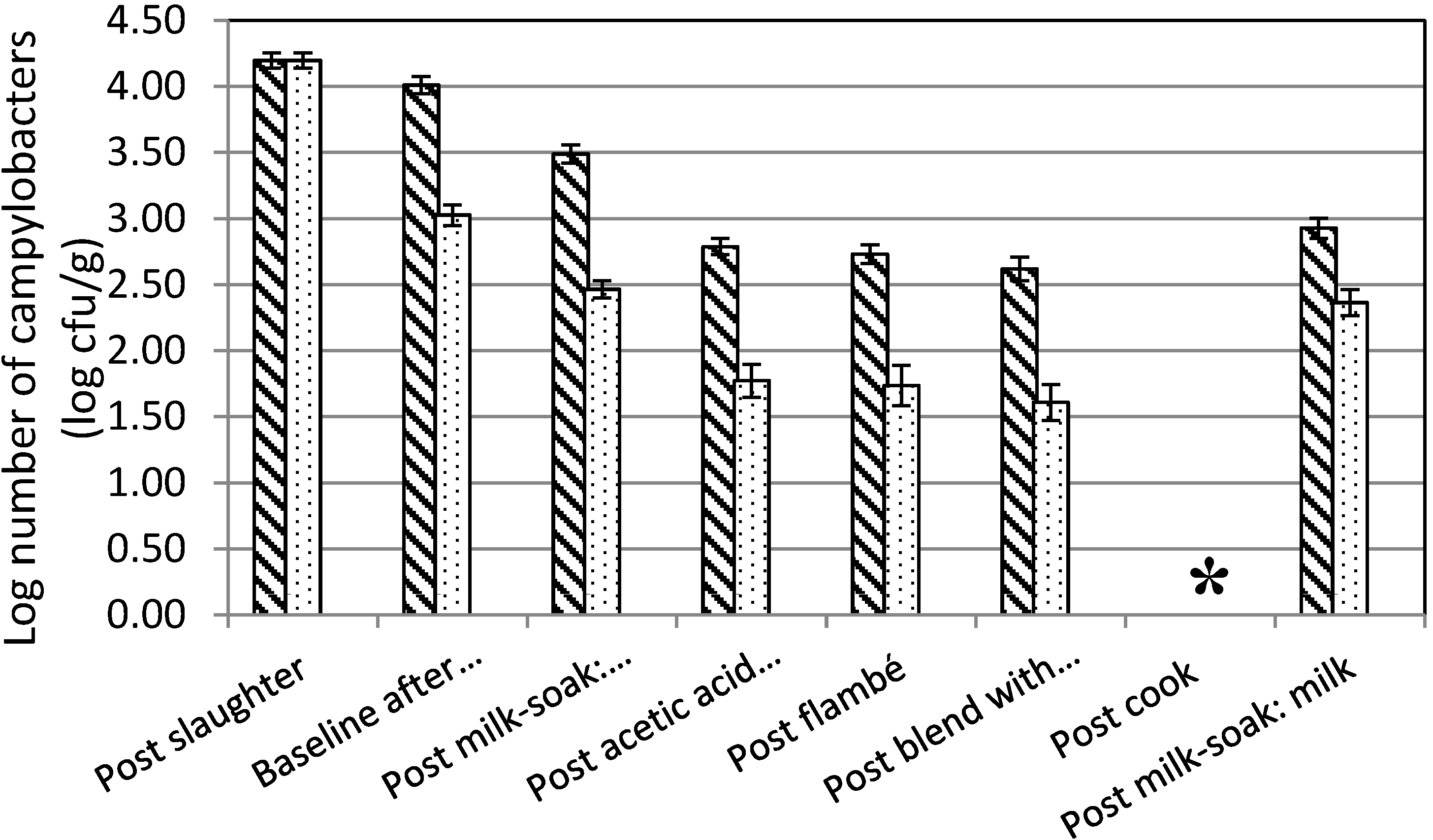
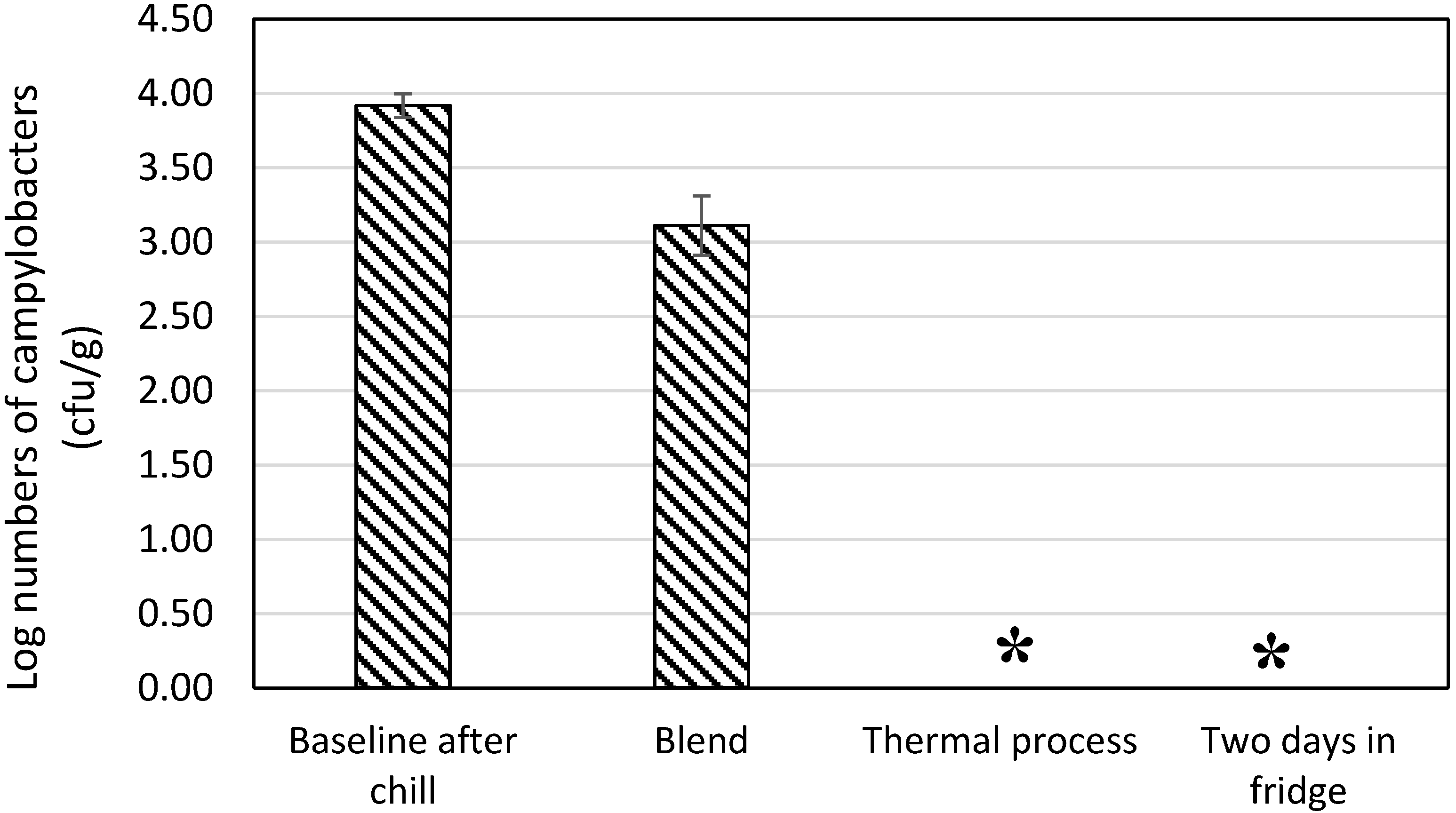

4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blaser, M.J. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 1997, 176, S103–S105. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.; Keenan, A.; Vivancos, R. Food-borne Campylobacter outbreak in Liverpool associated with cross-contamination from chicken liver parfait: Implications for investigation of similar outbreaks. Pub. Health 2012, 126, 657–659. [Google Scholar] [CrossRef]
- Wheeler, J.G.; Sethi, D.; Cowden, J.M.; Wall, P.G.; Tompkins, D.S.; Hudson, M.J.; Roderick, P.J.; The Infectious Intestinal Disease Executive. Study of infectious intestinal disease in England: Rates in the community, presenting to general practice, and reported to national surveillance. Brit. Med. J. 1999, 318. [Google Scholar] [CrossRef]
- European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015, 138. [Google Scholar] [CrossRef]
- Evans, S.J.; Sayers, A.R. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Prev. Vet. Med. 2000, 46, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Strachan, N.J.; MacRae, C.M.; Thomson, A.; Rotariu, O.; Ogden, I.D.; Forbes, K.J. Source attribution, prevalence and enumeration of Campylobacter spp. from retail liver. Int. J. Food Microbiol. 2012, 153, 234–236. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.C.; Harding, O.; Fisher, L.; Cowden, J. A continuous common-source outbreak of Campylobacteriosis associated with changes to the preparation of chicken liver pâté. Epidemiol. Infect. 2009, 137, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Little, C.L.; Gormley, F.J.; Rawal, N.; Richardson, J.F. A recipe for disaster: outbreaks of Campylobacteriosis associated with poultry liver pate in England and Wales. Epidemiol. Infect. 2010, 138, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.; Corry, J.E.; Tchórzewska, M.A.; Morris, V.K.; Hutchison, M.L. Freezing as an intervention to reduce the numbers of Campylobacters isolated from chicken livers. Lett. Appl. Microbiol. 2013, 57, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Grand, M.; Liniger, M.; Simmen, A. Campylobacter contaminations of poultry liver consequences for food handler and consumers. Arch. Lebensmittelhyg. 1995, 46, 11–12. [Google Scholar]
- Whyte, R.; Hudson, J.; Graham, C. Campylobacter in chicken livers and their destruction by pan frying. Let. Appl. Microbiol. 2006, 43, 591–595. [Google Scholar] [CrossRef]
- Matasovska, N.; Sladka, O.; Mraz, O.; Matyas, Z.; Tomancova, I. Campylobacter jejuni in slaughtered chickens from the viewpoint of food hygiene. Acta. Vet. Brno. 1992, 61, 61–67. [Google Scholar]
- Information Skills for Researchers. Available online: http://www.open.ac.uk/infoskills-researchers/evaluation-introduction.htm (accessed on 16 March 2015).
- Technical Committee. ISO/TC 34 “Food Products” of the International Organization for Standardization (ISO) Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors; International Organization for Standards: Geneva, Switzerland, 2012. [Google Scholar]
- Martins, P.; Sbaite, P.; Benites, C.; Maciel, M. Thermal Characterization of orange, lemongrass, and basil essential oils. In Chemical Engineering Transactions: 10th International Conference on Chemical and Process Engineering; Pierucci, S., Ed.; Italian Association for Chemical Engineering: Florence, Italy, 2011; pp. 463–468. [Google Scholar]
- De Jong, A.E.I.; van Asselt, E.D.; Zwietering, M.H.; Nauta, M.J.; de Jonge, R. Extreme heat resistance of food borne pathogens Campylobacter jejuni, Escherichia coli, and Salmonella Typhimurium. on chicken breast fillet during cooking. Int. J. Microbiol. 2012. [Google Scholar] [CrossRef]
- Heimann, P.; Jemmi, T. Campylobacteriose-risiko beim verzehr von hühnerleber in gastronomiebetrieben. In Proceedings of Arbeitstagung des Arbeitsgebietes Lebensmittelhygiene der Deutschen Veterinärmedizinischen Gesellschaft, Garmisch-Partenkirchen, Germany, 12-15 June 1995; 1995; pp. 127–132. (In German)[Google Scholar]
- Barot, M.S.; Mosenthal, A.C.; Bokkenheuser, V.D. Location of Campylobacter jejuni in infected chicken livers. J. Clin. Microbiol. 1983, 17, 921–922. [Google Scholar] [PubMed]
- Keum-Il, J.; Min-Gon, K.; Sang-Do, H.; Keun-Sung, K.; Kyu-Ho, L.; Duck-Hwa, C.; Cheorl-Ho, K.; Kwang-Yup, K. Morphology and adhesion of Campylobacter jejuni to chicken skin under varying conditions. J. Microbiol. Biotechnol. 2007, 17, 202–206. [Google Scholar] [PubMed]
- Hu, L.; McDaniel, J.P.; Kopecko, D.J. Signal transduction events involved in human epithelial cell invasion by Campylobacter jejuni 81–176. Microb. Pathogen. 2006, 40, 91–100. [Google Scholar] [CrossRef]
- Gagnon, G.A.; Slawson, R.M. An efficient biofilm removal method for bacterial cells exposed to drinking water. J. Microbiol. Meth. 1999, 34, 203–214. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- De Wet, P.M.; Rode, H.; Sidler, D.; Lastovica, A.J. Allicin: a possible answer to antibiotic resistant Campylobacter diarrhoeal infection? Arch. Dis. Childhood 1999, 81. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Shallot (Allium ascalonicum L.) oil: Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria. Afr. J. Microbiol. Res. 2009, 3, 747–750. [Google Scholar]
- Venkitanarayanan, K.; Kollanoor-Johny, A.; Darre, M.; Donoghue, A.; Donoghue, D. Use of plant-derived antimicrobials for improving the safety of poultry products. Poult. Sci. 2013, 92, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Selgas, D.; Marin, M.L.; Pin, C.; Casas, C. Attachment of bacteria to meat surfaces—A review. Meat Sci. 1993, 34, 265–273. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchison, M.; Harrison, D.; Richardson, I.; Tchórzewska, M. A Method for the Preparation of Chicken Liver Pâté that Reliably Destroys Campylobacters. Int. J. Environ. Res. Public Health 2015, 12, 4652-4669. https://doi.org/10.3390/ijerph120504652
Hutchison M, Harrison D, Richardson I, Tchórzewska M. A Method for the Preparation of Chicken Liver Pâté that Reliably Destroys Campylobacters. International Journal of Environmental Research and Public Health. 2015; 12(5):4652-4669. https://doi.org/10.3390/ijerph120504652
Chicago/Turabian StyleHutchison, Mike, Dawn Harrison, Ian Richardson, and Monika Tchórzewska. 2015. "A Method for the Preparation of Chicken Liver Pâté that Reliably Destroys Campylobacters" International Journal of Environmental Research and Public Health 12, no. 5: 4652-4669. https://doi.org/10.3390/ijerph120504652





