Estimation of Seasonal Risk Caused by the Intake of Lead, Mercury and Cadmium through Freshwater Fish Consumption from Urban Water Reservoirs in Arid Areas of Northern Mexico
Abstract
:1. Introduction
2. Experimental Section
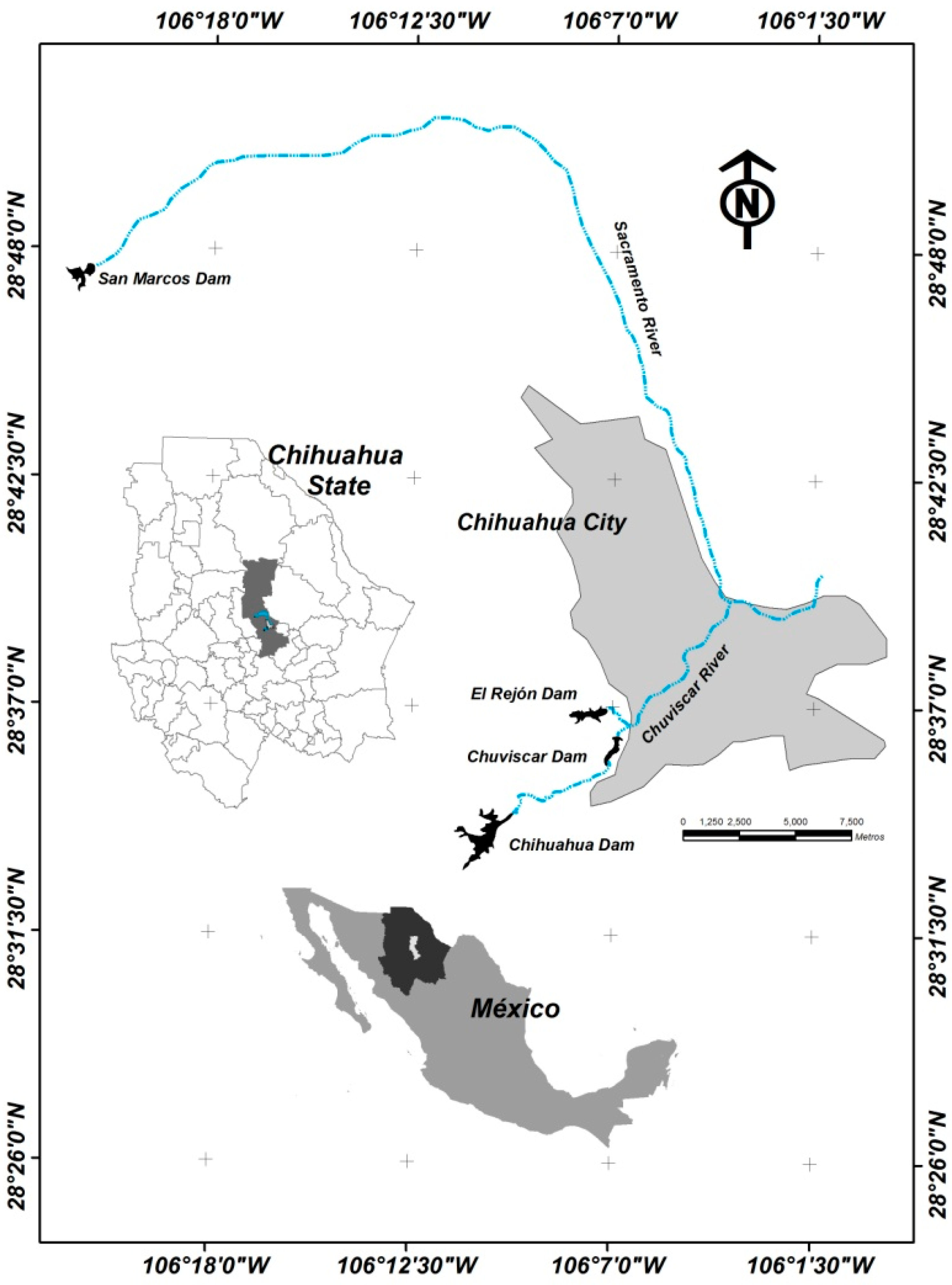
2.1. Study Area
2.2. Sampling
2.3. Reagents
2.4. Apparatus
2.5. Sample Pre-Treatment
2.6. Statistical Analysis
2.7. Estimated Weekly Intake
3. Results and Discussion
3.1. Method Validation
| Metal | Certified Value (mg/kg) | Obtained Value * (mg/kg) | Recovery (%) |
|---|---|---|---|
| Mercury a | 3.37 ± 0.14 | 3.35 ± 0.09 | 99.4 |
| Lead b | 0.32 ± 0.05 | 0.31 ± 0.03 | 96.8 |
| Cadmium b | 19.4 ± 0.6 | 19.34 ± 0.55 | 99.6 |
3.2. Metals Concentrations
| Water Reservoirs | Species | Sampling Season | Cadmium (mg/kg Wet Weight) | Lead (mg/kg Wet Weight) | Mercury (mg/kg Wet Weight) |
|---|---|---|---|---|---|
| El Rejón | Channel catfish | Spring | 0.061 ± 0.013a | 2.327 ± 0.080b | 0.041 ± 0.022 |
| Summer | 0.235 ± 0.013b | <0.042a | 0.039 ± 0.022 | ||
| Autumn | <0.0025a | 3.154 ± 0.080c | 0.059 ± 0.022 | ||
| Winter | <0.0025a | <0.042a | 0.047 ± 0.022 | ||
| Green sunfish | Spring | 0.027 ± 0.014 | 1.718 ± 0.560a,b | <0.005a | |
| Summer | <0.0025 | <0.042a | 0.098 ± 0.012b | ||
| Autumn | <0.0025 | 3.516 ± 0.560b | 0.059 ± 0.012a,b | ||
| Winter | <0.0025 | <0.042a | 0.034 ± 0.012a,b | ||
| Chihuahua | Channel catfish | Spring | <0.0025 | 0.729 ± 0.103b | <0.005a |
| Summer | 0.010 ± 0.007 | <0.042a | 0.047 ± 0.010a,b | ||
| Autumn | 0.024 ± 0.007 | 2.110 ± 0.103c | 0.042 ± 0.010a,b | ||
| Winter | <0.0025 | <0.042a | 0.089 ± 0.010b | ||
| Green sunfish | Spring | <0.0025 | 2.746 ± 0.087b | <0.005a | |
| Summer | 0.121 ± 0.055 | <0.042a | 0.465 ± 0.256b | ||
| Autumn | <0.0025 | 1.525 ± 0.087b | <0.005a | ||
| Winter | <0.0025 | <0.042a | <0.005a | ||
| San Marcos | Blue gill | Spring | <0.0025a | 0.623 ± 0.329 | 0.744 ± 0.119 |
| Summer | 0.036 ± 0.0004b | <0.042 | 0.333 ± 0.119 | ||
| Autumn | <0.0025a | 0.976 ± 0.329 | 0.395 ± 0.119 | ||
| Winter | <0.0025a | <0.042 | 0.275 ± 0.119 | ||
| Chuviscar | Channel catfish | Spring | 0.090 ± 0.005b | 4.298 ± 0.626b | <0.005 |
| Autumn | <0.0025a | <0.042a | 0.013 ± 0.004 | ||
| Winter | <0.0025a | <0.042a | <0.005 |
3.2.1. Mercury
3.2.2. Cadmium
3.2.3. Lead
3.3. Estimation of Weekly Intakes of Hg, Cd and Pb through Fish Consumption
| Water Reservoir | Species | Sampling Season | Cadmium | Lead | Mercury |
|---|---|---|---|---|---|
| El Rejón | Channel catfish | Spring | 0.199 | 7.602 | 0.134 |
| Summer | 0.768 | NA | 0.127 | ||
| Autumn | NA | 10.303 | 0.193 | ||
| Winter | NA | NA | 0.154 | ||
| Green sunfish | Spring | 0.088 | 5.612 | NA | |
| Summer | NA | NA | 0.320 | ||
| Autumn | NA | 11.486 | 0.193 | ||
| Winter | NA | NA | 0.111 | ||
| Chihuahua | Channel catfish | Spring | NA | 2.381 | NA |
| Summer | 0.033 | NA | 0.154 | ||
| Autumn | 0.078 | 6.893 | 0.137 | ||
| Winter | NA | NA | 0.291 | ||
| Green sunfish | Spring | NA | 8.970 | NA | |
| Summer | 0.395 | NA | 1.519 | ||
| Autumn | NA | 4.982 | NA | ||
| Winter | NA | NA | NA | ||
| San Marcos | Blue gill | Spring | NA | 2.035 | 2.430 |
| Summer | 0.118 | NA | 1.088 | ||
| Autumn | NA | 3.188 | 1.290 | ||
| Winter | NA | NA | 0.898 | ||
| Chuviscar | Channel catfish | Spring | 0.294 | 14.040 | NA |
| Autumn | NA | NA | 0.042 | ||
| Winter | NA | NA | NA |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Food Safety Authority (EFSA). Opinion of the scientific panel on contaminants in the food chain on a request from the European parliament related to the safety assessment of wild and farmed fish. EFSA J. 2005, 236, 1–118. [Google Scholar]
- Environmental Protection Agency (EPA). Aquatic Biota, Fate and Transport. Available online: http://www.epa.gov/risk/expobox/media/biota-ft.htm (accessed on 22 November 2013).
- Kamau, J.N.; Gachanja, A.; Ngila, C.; Kazungu, J.M.; Zhai, M. The seasonal influence on the spatial distribution of dissolved selected metals in Lake Naivasha Kenya. Phys. Chem. Earth 2014, 67–69, 111–116. [Google Scholar]
- Papafilippaki, A.K.; Kotti, M.E.; Stavroulakis, G.G. Seasonal variations in dissolved heavy metals in the Keritis River, Chania, Greece. Glob. NEST J. 2008, 10, 320–325. [Google Scholar]
- Audry, S.; Blanc, G.; Schafer, J. Cadmium transport in the Lot-Garonne River system (France)—Temporal variability and a model for flux estimation. Sci. Total Environ. 2004, 319, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.F.; Kurzweil, H.; Wruss, W.; Fenzl, N. Cadmium in the Amazonian Guajará Estuary: Distribution and remobilization. Environ. Pollut. 2006, 140, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Eckley, C.S.; Branfireun, B. Simulated rain events on an urban roadway to understand the dynamics of mercury mobilization in storm water runoff. Water Res. 2009, 43, 3635–3646. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Martorell, J.J.; Galindo-Riano, M.D.; García-Vargas, M.; Granado-Castro, M.D. Bioavailability of heavy metals monitoring water, sediments and fish species from a polluted estuary. J. Hazard. Mater. 2009, 162, 823–836. [Google Scholar] [CrossRef] [PubMed]
- SGM. Mexican Geological Survey, Lead, Arsenic and Cadmium Geochemistry Chihuahua Letters (H13-C66). 2002. Available online: http://mapserver.sgm.gob.mx/cartas_impresas/productos/cartas/cartas50/geoquimica50/zn/numcarta50_zn.html (accessed on 3 August 2014).
- Gunawardena, J.; Egodawatta, P.; Ayoko, G.A.; Goonetilleke, A. Atmospheric deposition as a source of heavy metals in urban stormwater. Atmos. Environ. 2013, 68, 235–242. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Castro-González, M.I.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environmental Programme (UNEP). Lead & Cadmium—Priorities for Action. Available online: http://www.unep.org/chemicalsandwaste/Home/tabid/197/chemicalsandwaste/LeadCadmium/tabid/29372/Default.aspx (accessed on 14 July 2014).
- European Food Safety Authority (EFSA). Cadmium dietary exposure in the European population. EFSA J. 2012, 10. [Google Scholar] [CrossRef]
- Benefice, E.; Luna, S.; López, R. Fishing activity, health characteristics and mercury exposure of Amerindian women living alongside the Beni River (Amazonian Bolivia). Int. J. Hyg. Environ. Health 2010, 213, 458–464. [Google Scholar] [CrossRef] [PubMed]
- NOM-027-SSA1-1993, Goods and Services. Fishery Products. Fresh Fish, Fresh and Frozen. Sanitary Specifications. Available online: http://www.conapesca.sagarpa.gob.mx/wb/cona/nom_ssa (accessed on 11 July 2014).
- Commission Regulation (EC) No. 1881/2006, Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: http://faolex.fao.org/cgi-bin/faolex.exe?rec_id=052610&database=faolex&search_type=link&table=result&lang=eng&format_name=@ERALL (accessed on 19 December 2006).
- CODEX Alimentarius Commission. Report of the 21st Session of the Codex ALINORM 89/12A; Committee on Food Additives and Contaminants: Geneva, Switzerland, 1989. [Google Scholar]
- CODEX Alimentarius Commission. Report of the 33rd Session of the Codex ALINORM 01/12A; Committee on Food Additives and Contaminants: Geneva, Switzerland, 2001. [Google Scholar]
- World Health Organization (WHO). Human Health Risk Assessment Toolkit: Chemical Hazards. 2010. Available online: http://www.inchem.org/documents/harmproj/harmproj/harmproj8.pdf (accessed on 2 June 2014).
- World Health Organization (WHO). Guidelines for Drinking-Water Quality 2004, 3rd ed.; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization (WHO). Cadmium in Drinking-Water, 2011. Available online: http://www.who.int (accessed on 20 April 2014).
- Nevárez, M.; Moreno, M.V.; Sosa, M.; Bundschuh, J. Arsenic in freshwater fish in the Chihuahua County water reservoirs (Mexico). J. Environ. Sci. Health Part A 2011, 46, 1283–1287. [Google Scholar] [CrossRef]
- Leal, L.O.; Elsholz, O.; Forteza, R.; Cerda, V. Determination of mercury by multisyringe flow injection system with cold-vapor atomic absorption spectrometry. Anal. Chim. Acta 2006, 573, 399–405. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Statistics, Geography and Informatics (INEGI). Synthesis of Geographic Information of the State of Chihuahua, 1st ed.; Instituto Nacional de Estadística, Geografía e Informática: Ciudad de México, México, 2003. [Google Scholar]
- U.S. EPA. Fish sampling and analysis. In Environmental Protection Agency, Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories 2000, 3rd ed.; EPA: Washington, DC, USA, 2000; Volume 1. [Google Scholar]
- Secretariat of Agriculture, Livestock, Rural Development, Fisheries and Food (SAGARPA). Fisheries Statistical Yearbook 2002. Available online: http://www.fao.org/fishery/countrysector/naso_mexico/es (accessed on 7 April 2014).
- Olaiz, G.; Rivera, J.; Shamah, T.; Rojas, R.; Villalpando, S.; Hernández, M.; Sepúlveda, J. National Survey of Health and Nutrition 2006; National Public Health Institute: Cuernavaca, Mexico, 2006. [Google Scholar]
- Pal, M.; Ghosh, M. Relationship of methyl mercury accumulation with lipid and weight in two river cat fish species, Wallagoo attu and Mystus aor, from West Bengal, India. Environ. Monit. Assess. 2013, 185, 31–37. [Google Scholar] [CrossRef] [PubMed]
- PNUMA; United Nations Environment Programme (UNEP). Chemicals, Global Mercury Assessment; UNEP: Geneva, Switzerland, 2005. [Google Scholar]
- World Health Organization (WHO). Evaluation of Certain Contaminants in Food. Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series; No. 959; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Ferriz, H. Geology of the San Marcos cauldron, Chihuahua. Univ. Nal. Autóo. México Inst. Geol. 1981, 5, 65–79. [Google Scholar]
- Guinot, D.; Ureña, R.; Pastor, A.; Varó, I.; Ramo, J.; Torreblanca, A. Long-term effect of temperature on bioaccumulation of dietary metals and metallothionein induction in Sparus aurata. Chemosphere 2012, 87, 1215–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baykan, U.; Atli, G.; Canli, M. The effects of temperature and metal exposures on the profiles of metallothionein-like proteins in Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2007, 23, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Comisión Nacional del Agua (CNA). Volumen de Almacenamiento de Las Presas Chihuahua y El Rejon; Technical report; CNA: Chihuahua, Chih., Mexico, 2008. [Google Scholar]
- De Conto Cinier, C.; Petit-Ramel, M.; Faure, R.; Garin, D. Kinetics of cadmium accumulation and elimination in carp Cyprinus carpio tissues. Comp. Biochem. Physiol. Part C 1999, 122, 345–352. [Google Scholar]
- Sana, B.; Imed, M.; Tmim, D.; Khaled, S.; Abdelhamid, K. Cadmium bioaccumulation in three benthic fish species, Salaria basilisca, Zosterisessor ophiocephalus and Solea vulgaris collected from the Gulf of Gabes in Tunisia. J. Environ. Sci. 2009, 21, 980–984. [Google Scholar] [CrossRef]
- Cao, L.; Huang, W.; Shana, X.; Yed, Z.; Doua, S. Tissue-specific accumulation of cadmium and its effects on antioxidative responses in Japanese flounder juveniles. Environ. Toxicol. Pharmacol. 2012, 33, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Luck, J.M.; Othman, D.B. Trace element and Pb isotope variability during rainy events in the NW Mediterranean: Constraints on anthropogenic and natural sourcesm. Chem. Geol. 2002, 182, 443–460. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, Y.S.; Ki, S.J.; Lee, Y.G.; Cha, S.M.; Cho, K.H.; Kim, J.H. Characteristics of wet and dry weather heavy metal discharges in the Yeongsan Watershed, Korea. Sci. Total Environ. 2009, 407, 3482–3493. [Google Scholar] [CrossRef] [PubMed]
- Outridge, P.M.; Hermanson, M.H.; Lockhart, W.L. Regional variations in atmospheric deposition and sources of anthropogenic lead in lake sediments across the Canadian Arctic. Geochim. Cosmochim. Acta 2002, 66, 3521–3531. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATDSR). Toxicological Profile for Lead; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2007. Available online: http://www.atsdr.cdc.gov/ToxProfiles/tp13.pdf (accessed on 3 January 2014).
- Castillo, S.; De la Rosa, J.D.; Sánchez, A.M.; González, Y.; Fernández, J.C.; González, I.; Romero, A. Contribution of mine wastes to atmospheric metal deposition in the surrounding area of an abandoned heavily polluted mining district (Rio Tinto mines, Spain). Sci. Total Environ. 2013, 449, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Mexican Geological Survey (SGM). Statistical Yearbook of Mexican Mining, 2011. Available online: http://www.sgm.gob.mx/index.php?option=com_content&task=view&id=59&Itemid=67 (accessed on 15 April 2014).
- World Health Organization (WHO). Environmental Health Criteria 85. Lead—Environmental Aspects, 1989. Available online: http://www.inchem.org/documents/ehc/ehc/ehc85.htm#SectionNumber:1.1 (accessed on 15 April 2014).
- Eyckmans, M.; Lardon, I.; Wood, C.M.; Boeck, G. Physiological effects of waterborne lead exposure in spiny dogfish (Squalus acanthias). Aquat. Toxicol. 2013, 126, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ay, Ö.; Kalay, M.; Tamer, L.; Canli, M. Copper and lead accumulation in tissues of a freshwater fish tilapia zillii and its effects on the branchial Na,K-atpase activity. Environ. Contam. Toxicol. 1999, 62, 160–168. [Google Scholar] [CrossRef]
- Wagner, A.; Boman, J. Biomonitoring of trace elements in muscle and liver tissue of freshwater fish. Spectrochim. Acta Part B 2003, 58, 2215–2226. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Fisheries Circulars No. 821 Fish and Fishery Products—World Apparent Consumption Statistics based on Food Balance Sheets (1961-) and CD Dissemination of FAO Yearbook of Fishery Statistics 2007 and After, 2013. Available online: http://www.fao.org/fishery/statistics/global-consumption/en (accessed on 4 September 2014).
- Vieira, C.; Morais, S.; Ramos, S.; Delerue-Matos, C.; Oliveira, M.B.P.P. Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: Intra- and inter-specific variability and human health risks for consumption. Food Chem. Toxicol. 2011, 49, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Antonijevic, B.; Jankovic, S.; Curcic, M.; Durgo, K.; Stokic, E.; Srdic, B.; Tomic-Naglic, D. Risk characterization for mercury, dichlorodiphenyltrichloroethane and polychlorinated biphenyls associated with fish consumption in Serbia. Food Chem. Toxicol. 2011, 49, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: Estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem. Toxicol. 2008, 46, 2782–2788. [Google Scholar] [CrossRef] [PubMed]
- Herreros, M.A.; Iñigo-Nuñez, S.; Sanchez-Perez, E.; Encinas, T.; Gonzalez-Bulnes, A. Contribution of fish consumption to heavy metals exposure in women of childbearing age from a Mediterranean country (Spain). Food Chem. Toxicol. 2008, 46, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). El mercurio y la salud. Nota Descriptiva N° 361, 2013. Available online: http://www.who.int/mediacentre/factsheets/fs361/es/ (accessed on 20 August 2014).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevárez, M.; Leal, L.O.; Moreno, M. Estimation of Seasonal Risk Caused by the Intake of Lead, Mercury and Cadmium through Freshwater Fish Consumption from Urban Water Reservoirs in Arid Areas of Northern Mexico. Int. J. Environ. Res. Public Health 2015, 12, 1803-1816. https://doi.org/10.3390/ijerph120201803
Nevárez M, Leal LO, Moreno M. Estimation of Seasonal Risk Caused by the Intake of Lead, Mercury and Cadmium through Freshwater Fish Consumption from Urban Water Reservoirs in Arid Areas of Northern Mexico. International Journal of Environmental Research and Public Health. 2015; 12(2):1803-1816. https://doi.org/10.3390/ijerph120201803
Chicago/Turabian StyleNevárez, Myrna, Luz O. Leal, and Myriam Moreno. 2015. "Estimation of Seasonal Risk Caused by the Intake of Lead, Mercury and Cadmium through Freshwater Fish Consumption from Urban Water Reservoirs in Arid Areas of Northern Mexico" International Journal of Environmental Research and Public Health 12, no. 2: 1803-1816. https://doi.org/10.3390/ijerph120201803
APA StyleNevárez, M., Leal, L. O., & Moreno, M. (2015). Estimation of Seasonal Risk Caused by the Intake of Lead, Mercury and Cadmium through Freshwater Fish Consumption from Urban Water Reservoirs in Arid Areas of Northern Mexico. International Journal of Environmental Research and Public Health, 12(2), 1803-1816. https://doi.org/10.3390/ijerph120201803





