Growth and Heavy Metal Accumulation of Koelreuteria Paniculata Seedlings and Their Potential for Restoring Manganese Mine Wastelands in Hunan, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design and Growth Media
2.3. Seedling Growth Measurements and Chemical Analysis
2.4. Data Analysis
3. Results
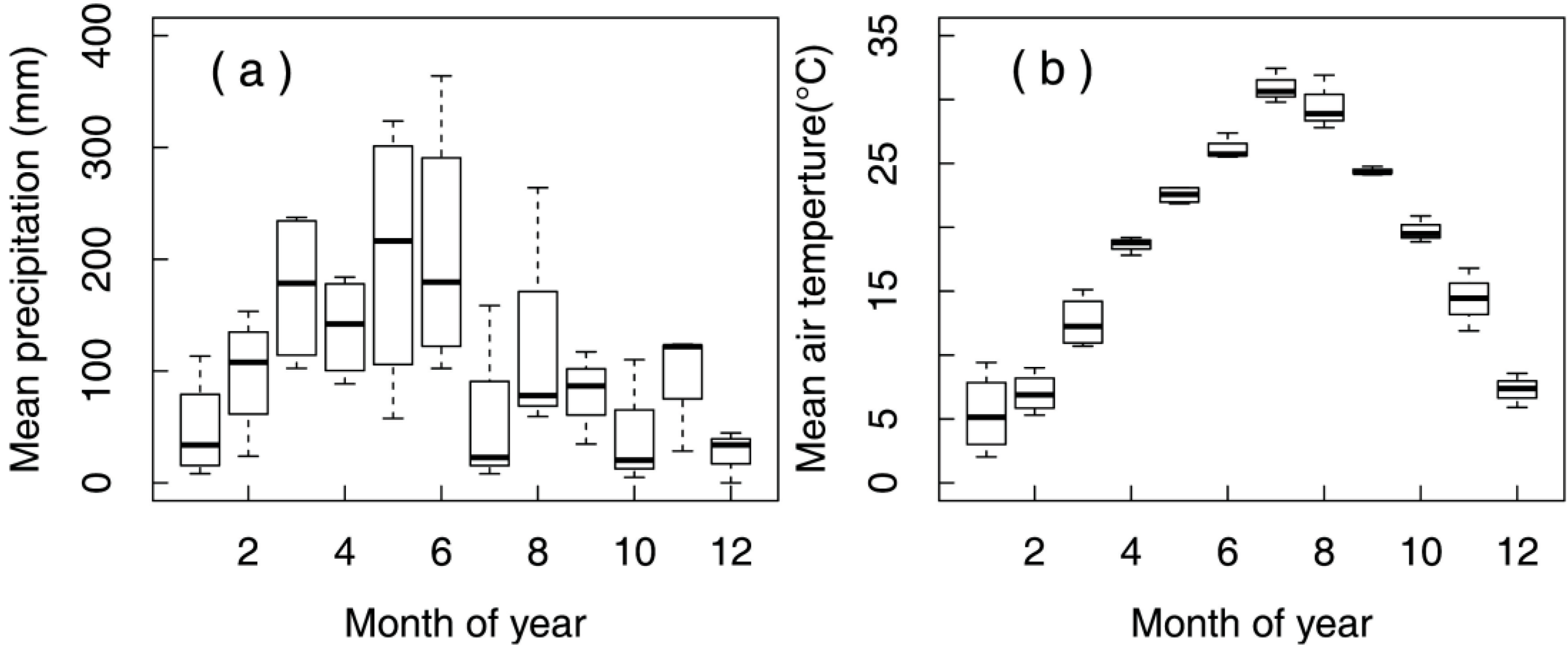
3.1. Chemical Characteristics of Mine Wastes and Soil
3.2. Survival Rate and Fine Root Development
| Samples | pH | Bulk Density (g∙cm−3) | Total N (g∙kg−1) | Total P (g∙kg−1) | Total K (g∙kg−1) | Fe (mg∙kg−1) | Zn (mg∙kg−1) | Mn (mg∙kg−1) |
|---|---|---|---|---|---|---|---|---|
| C | 4.29 | 1.37 | 1.200 ± 0.082 a | 0.606 ± 0.046 b | 0.029 ± 0.001 a | 11,803.1 ± 69.1 b | 51.7 ± 9.6 c | 97.6 ± 16.3 c |
| T | 9.07 | 1.44 | 0.575 ± 0.150 b | 0.359 ± 0.344 b | 0.001 ± 0.001 b | 2396.7 ± 17.3 c | 61.0 ± 42.9 c | 13,178.3 ± 77.5 b |
| S | 6.56 | 1.64 | 1.275 ± 0.050 a | 4.083 ± 0.281 a | 0.018 ± 0.003 b | 17,185.7 ± 334.4 a | 170.0 ± 12.5 a | 17,391.4 ± 64.8 a |
| ST | 8.37 | 1.54 | 0.900 ± 0.000 b | 3.462 ± 0.062 a | 0.015 ± 0.001 b | 13,292.0 ± 5.4 b | 135.28.4 b | 16,484.0 ± 124.5 a |
| Background value (1) | − | − | − | − | − | 34,000 | 94.9 | 320 |
| Grade II (2) | − | − | − | − | − | − | 200 | − |

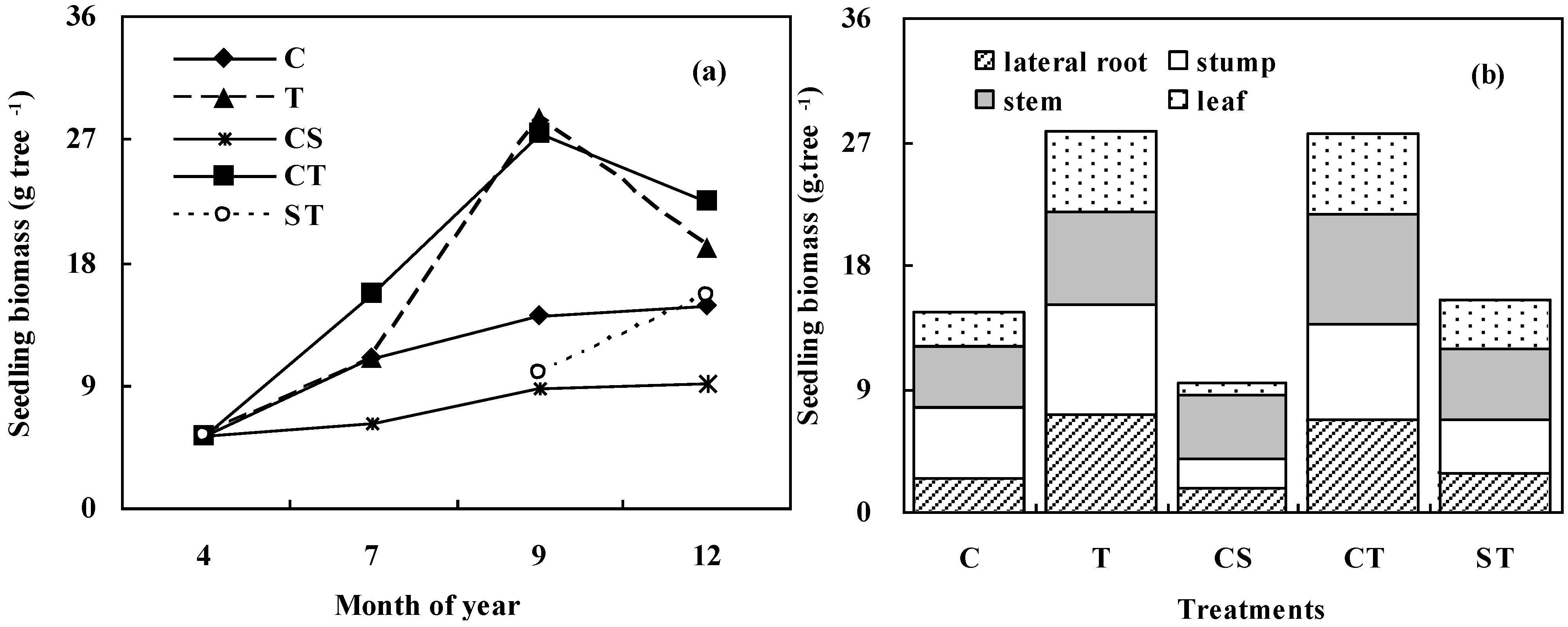
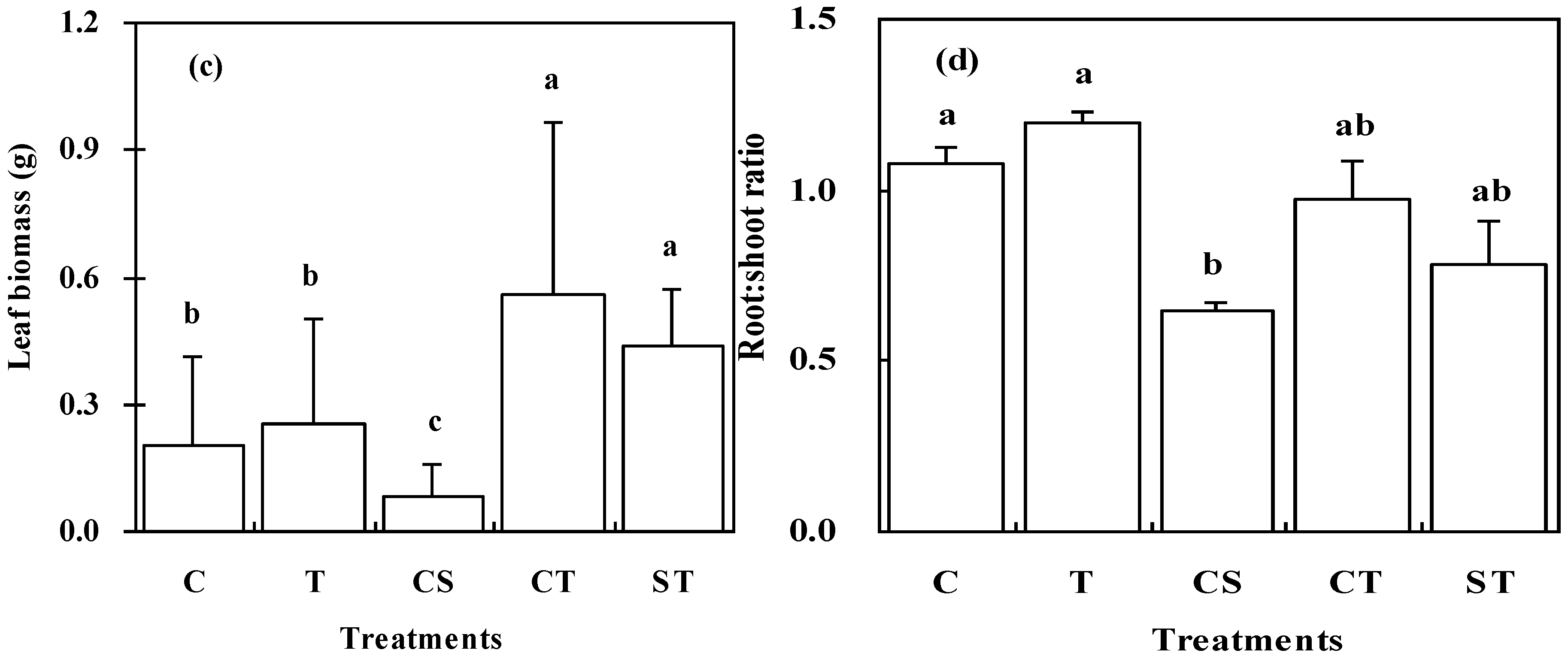
3.3. Biomass Allocation of K. Paniculata Seedlings

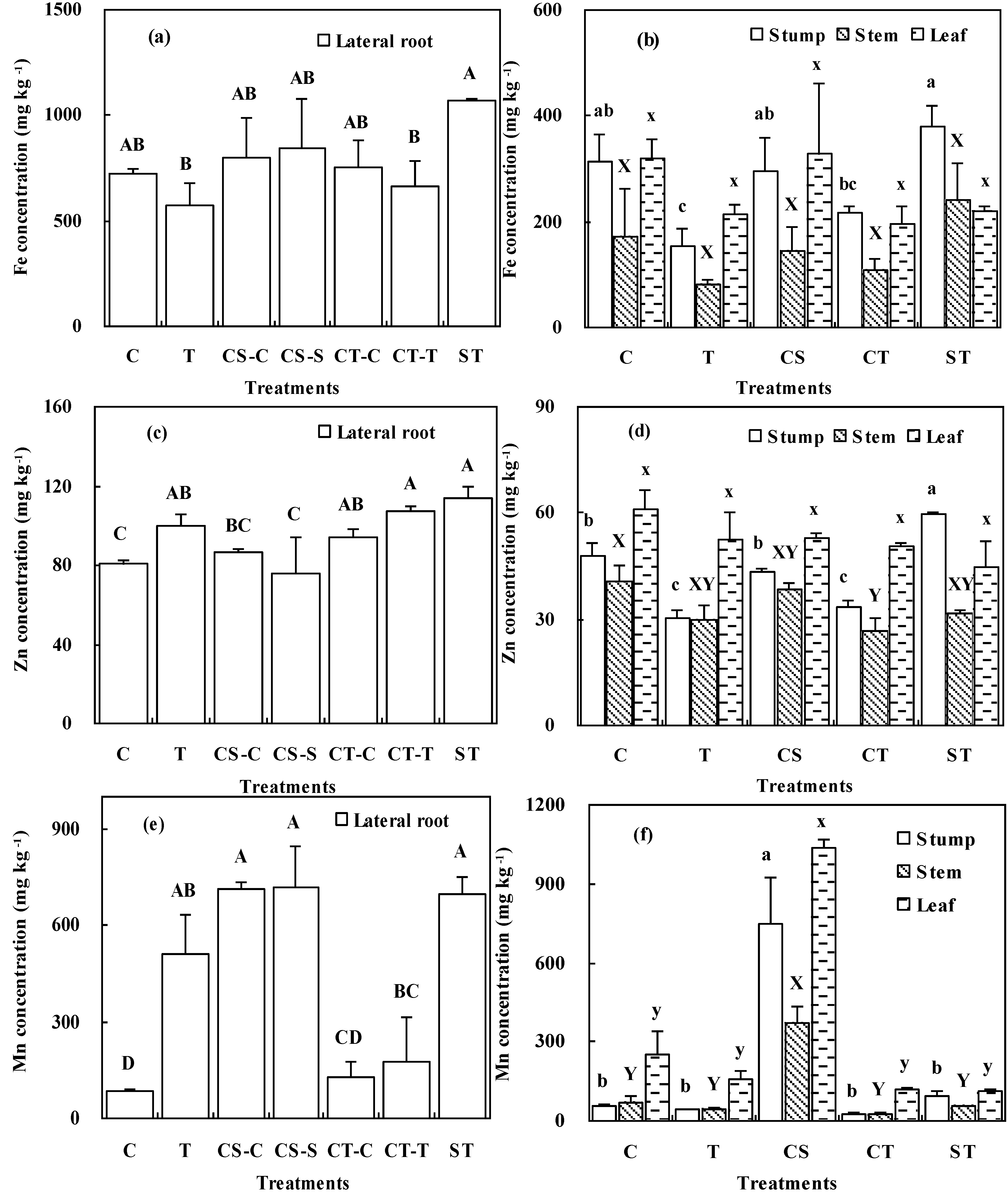
3.4 Heavy Metal Accumulation and Translocation
| Treatments | Fe (mg) | Zn (mg) | Mn (mg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Above-Ground | Below-Ground | Total | Above-Ground | Below-Ground | Total | Above-Ground | Below-Ground | Total | |
| C | 0.595 | 3.345 | 3.94 | 0.238 | 0.484 | 0.722 | 0.447 | 0.508 | 0.955 |
| T | 0.821 | 2.595 | 3.416 | 0.318 | 0.608 | 0.926 | 0.599 | 2.527 | 3.126 |
| ST | 1.58 | 3.695 | 5.275 | 0.283 | 0.523 | 0.806 | 0.668 | 1.588 | 2.256 |
| CT | 1.044 | 4.009 | 5.053 | 0.31 | 0.661 | 0.971 | 0.484 | 0.89 | 1.374 |
| CS | 0.563 | 1.68 | 2.243 | 0.198 | 0.211 | 0.409 | 2.59 | 3.414 | 6.004 |
4. Discussion
4.1. Differences in Characteristics of Mine Wastes and Soil
4.2. Influences on Survival Rate and Fine Root Development
4.3. Difference in Biomass Allocation of K. Paniculata Seedlings
4.4. Heavy Metal Accumulation and Translocation
| Treatments | BCF (Mean ± SE) | TF (Mean ± SE) | |||||
|---|---|---|---|---|---|---|---|
| Fe | Zn | Mn | Fe | Zn | Mn | ||
| C | 0.03 (0.01) | 1.39 (0.07) | 3.12 (0.10) | 0.44 (0.11) | 0.76 (0.09) | 2.54 (0.34) | |
| T | 0.12 (0.10) | 1.18 (0.15) | 0.01 (0.02) | 0.37 (0.09) | 0.53 (0.14) | 0.19 (0.21) | |
| CT | -C | 0.02 (0.00) | 1.11 (0.11) | 0.25 (0.13) | 0.26 (0.17) | 0.54 (0.02) | 0.71 (0.08) |
| -T | − | 2.90 (0.20) | 0.01 (0.02) | − | 0.47 (0.02) | − | |
| S | − | − | − | − | − | − | |
| CS | -C | 0.03 (0.00) | 1.04 (0.08) | 1.38 (0.10) | 0.41 (0.39) | 0.61 (0.02) | 1.41 (0.04) |
| -S | − | 0.32 (0.03) | 0.06 (0.01) | − | 0.70 (0.02) | − | |
| ST | 0.02 (0.01) | 0.34 (0.05) | 0.01 (0.01) | 0.21 (0.05) | 0.39 (0.10) | 0.15 (0.10) | |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Santamaria, A.B.; Cushing, C.A.; Antonini, J.M.; Finley, B.L.; Mowat, F.S. State-of-the-science review: Does manganese exposure during welding pose a neurological risk? J. Toxicol. Environ. Health B Crit. Rev. 2007, 10, 417–465. [Google Scholar] [CrossRef]
- Palacios, N.; Fitzgerald, K.; Roberts, A.L.; Hart, J.E.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A.; Laden, F. A prospective analysis of airborne metal exposures and risk of Parkinson disease in the nurses’ health study cohort. Environ. Health Perspect. 2014, 122, 933–938. [Google Scholar]
- Wang, X.L.; Sato, T.; Xing, B.S.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. The contribution of systematic review and meta-analysis methods to human health risk assessment: Neurobehavioral effects of manganese. Hum. Ecol. Risk Assess. 2008, 14, 1250–1272. [Google Scholar] [CrossRef]
- Haynes, E.N.; Heckel, P.; Ryan, P.; Roda, S.; Leung, Y.K.; Sebastian, K.; Succop, P. Environmental manganese exposure in residents living near a ferromanganese refinery in Southeast Ohio: A pilot. Neurotoxicology 2010, 31, 468–474. [Google Scholar] [CrossRef]
- Santamaria, A.B.; Sulsky, S.I. Risk assessment of an essential element: Manganese. J. Toxicol. Environ. Health A 2010, 73, 128–155. [Google Scholar] [CrossRef] [PubMed]
- Aboud, A.A.; Tidball, A.M.; Kumar, K.K.; Neely, M.D.; Ess, K.C.; Erikson, K.M.; Bowman, A.B. Genetic risk for Parkinson’s disease correlates with alterations in neuronal manganese sensitivity between two human subjects. Neurotoxicology 2012, 33, 1443–1449. [Google Scholar] [CrossRef]
- Kim, K.K.; Kim, K.W.; Kim, J.Y.; Kim, I.S.; Cheong, Y.W.; Min, J.S. Characteristics of tailings from the closed metal mines as potential contamination source in South Korea. Environ. Geol. 2001, 41, 358–364. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ma, Z.W.; van der Kuijp, T.J.; Yuan, Z.W.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Winder, B.S. Manganese in the air: Are children at greater risk than adults? J. Toxicol. Environ. Health A 2010, 73, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Lu, H.; Li, Z.; Zou, B.; McBride, M.B. Multiple exposure and effects assessment of heavy metals in the population near mining area in south China. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.M.; Liang, J.; Guo, S.L.; Shi, L.; Xiang, L.; Li, X.; Du, C. Spatial analysis of human health risk associated with ingesting manganese in Huangxing Town, middle China. Chemosphere 2009, 77, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.; Wright, R.; Amarasiriwardena, C.; Bellinger, D. A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 2002, 110, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Li, A.M.; Wang, Q.J.; Zhou, Y.; Fu, L.C.; Li, Y. Assessment of the manganese content of the drinking water source in Yancheng, China. J. Hazard. Mater. 2010, 182, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Y.; Wang, Z.X.; Wang, Y.Y.; Yang, Z.H.; Wang, H.Y.; Wu, X. Ingestion risks of metals in groundwater based on TIN model and dose-response assessment—A case study in the Xiangjiang watershed, central-south China. Sci. Total Environ. 2010, 408, 3118–3124. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Factor-Litvak, P.; Wasserman, G.A.; Liu, X.; Ahmed, E.; Parvex, F.; Slavkovich, V.; Levy, D.; Mey, J.; van Geen, A.; et al. Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ. Health Perspect. 2011, 119, 1501–1506. [Google Scholar] [CrossRef]
- Riojas-Rodríguez, H.; Solís-Vivanco, R.; Schilmann, A.; Montes, S.; Rodríguez, S.; Ríos, C.; Rodríguez-Agudelo, Y. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ. Health Perspect. 2010, 118, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Cherry, N.; Shaik, K.; McDonald, C.; Chowdhury, Z. Manganese, arsenic, and infant mortality in Bangladesh: An ecological analysis. Arch. Environ. Occup. Health 2010, 65, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Hafeman, D.; Factor-Litvak, P.; Cheng, Z.; van Geen, A.; Ahsan, H. Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environ. Health Perspect. 2007, 115, 1107–1112. [Google Scholar] [CrossRef]
- Li, M.S. Ecological restoration of mineland with particular reference to the metalliferous mine wasteland in China: A review of research and practice. Sci. Total Environ. 2006, 357, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Singh, S.P. A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J. Energ. Environ. 2005, 6, 214–231. [Google Scholar]
- Liu, L. Made in China: Cancer villages. Environ. Sci. Policy Sustain. Dev. 2010, 52, 8–21. [Google Scholar] [CrossRef]
- Badr, N.; Fawzy, M.; Al-Qahtani, K.M. Phytoremediation: An ecological solution to heavy-metal-polluted soil and evaluation of plant removal ability. World Appl. Sci. J. 2012, 16, 1292–1301. [Google Scholar]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Ann. Rev. Plant Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I. Phytoextraction: A cost-effective plant-based technology for the removal of metals from the environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.B.A.N.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The use of plants to remove heavy metals from soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Landberg, T.; Greger, M. Can heavy metal tolerant clones of Salix be used as vegetation filters on heavy metal contaminated land? In Proceedings of the Willow Vegetation Filters for Municipal Wastewaters and Sludges—A Biological Purification System, Swedish University of Agricultural Sciences, Uppsala, Sweden, 10 June 1994; pp. 133–144.
- Xue, S.G.; Chen, Y.X.; Baker, A.J.M.; Reeves, R.D.; Xu, X.H.; Lin, Q. Manganese uptake and accumulation by two populations of Phytolacca acinosa Roxb. (Phytolaccaceae). Water Air Soil Pollut. 2005, 160, 3–14. [Google Scholar] [CrossRef]
- Mertens, J.; Nevel, L.; van Schrijver, A.; Piesschaert, F.; Oosterbaan, A.; Tack, F.M.; Verheyen, K. Tree species effect on the redistribution of soil metals. Environ. Pollut. 2007, 149, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.; Luo, Y.P.; Su, Z.Y. Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ. Pollut. 2007, 147, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Son, Y.; Rhoades, C.C.; Noh, N.J.; Koo, J.W.; Kim, J.G. Seedling growth and heavy metal accumulation of candidate woody species for revegetating Korean mine spoils. Restor. Ecol. 2008, 16, 702–712. [Google Scholar] [CrossRef]
- Volk, T.A.; Abrahamson, L.P.; Nowak, C.A.; Smart, L.B.; Tharakan, P.J.; White, E.H. The development of short-rotation willow in the northeastern United States for bioenergy and bioproducts, agroforestry and phytoremediation. Biomass Bioenerg. 2006, 30, 715–727. [Google Scholar] [CrossRef]
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bullock, P.; Gregory, P.J. Soils in the Urban Environment; Blackwell: Oxford, UK, 1991; pp. 87–118. [Google Scholar]
- Watmough, S.A.; Dickinson, N.M. Dispersal and mobility of heavy metals in relation to tree survival in an aerially contaminated woodland soil. Environ. Pollut. 1995, 90, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Yadav, S.K.; Thawale, P.R.; Singh, S.K.; Juwarkar, A.A. Growth of Jatropha curcas on heavy metal contaminated soil amended with industrial wastes and Azotobacter—A greenhouse study. Bioresour. Technol. 2008, 99, 2078–2082. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Zeng, G.; Chai, L.; Xiao, X.; Song, X.; Min, Z. Pedological characteristics of Mn mine tailings and metal accumulation by native plants. Chemosphere 2008, 72, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.L.; Zhu, F.; Yan, W.D.; Fang, X.; Xiang, W.H.; Deng, X.W.; Wang, G.J.; Peng, C.H. Heavy metal accumulation by panicled goldenrain tree (Koelreuteria paniculata) and common elaeocarpus (Elaeocarpus decipiens) in abandoned mine soils in southern China. J. Environ. Sci. 2009, 21, 340–345. [Google Scholar] [CrossRef]
- Institute of Soils, the Chinese Academy of Science. Physio-Chemical Analysis of Soils; Shanghai Science and Technology Press: Shanghai, China, 1980. [Google Scholar]
- China National Environmental Monitoring Center. Soil Environmental Background Value in the People’s Republic of China, 1st ed.; China Environmental Science Press: Beijing, China, 1990; pp. 330–382. [Google Scholar]
- Sofianska, E.; Michailidis, K. Heavy metals and arsenic concentrations in agricultural soils of western Drama Plain, Macedonia, Northern Greece. In Proceedings of the International Conference Protection and Restoration of the Environment XI, Thessaloniki, Greece, 29 June–3 July, 2012.
- De Vries, W.; Lofts, S.; Tipping, E.; Meili, M.; Groenenberg, J.E.; Schütze, G. Impact of soil properties on critical concentrations of cadmium, lead, copper, zinc and mercury in soil and soil solution in view of ecotoxicological effects. Rev. Environ. Contam. Toxicol. 2007, 191, 47–89. [Google Scholar] [PubMed]
- Hamiani, O.; Khalil, H.E.; Lounate, K.; Sirguey, C.; Hafidi, M.; Bitton, G.; Schwartz, C.; Boularbah, A. Toxicity assessment of garden soils in the vicinity of mining areas in Southern Morocco. J. Hazard. Mater. 2010, 177, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.J.; Clemente, R.; Bernal, M.P. Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 2004, 57, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kahle, H. Response of roots of trees to heavy metals. Environ. Exp. Bot. 1993, 33, 99–119. [Google Scholar] [CrossRef]
- Alva, A.K.; Huang, B.; Paramasivam, S. Soil pH affects copper fractionation and phytotoxicity. Soil Sci. Soc. AM. J. 2000, 64, 955–962. [Google Scholar] [CrossRef]
- De Kroon, H. How do roots interact? Science 2007, 318, 1562–1563. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Hernández-Madrigal, F.; Cervantes, C.; Ortiz-Castro, R.; Carreón-Abud, Y.; Martínez-Trujillo, M. Phosphate relieves chromium toxicity in Arabidopsis thaliana plants by interfering with chromate uptake. BioMetals 2014, 27, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Trujillo, M.; Méndez-Bravo, A.; Ortiz-Castro, R.; Hernández-Madrigal, F.; Ibarra-Laclette, E.; Ruiz-Herrera, L.F.; Long, T.A.; Cervantes, C.; Herrera-Estrella, L.; López-Bucio, J. Chromate alters root system architecture and activates expression of genes involved in iron homeostasis and signaling in Arabidopsis thaliana. Plant Mol. Biol. 2014, 86, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Doumett, S.; Lamperi, L.; Checchini, L.; Azzarello, E.; Mugnai, S.; Mancuso, S.; Petruzzelli, M.; Del Bubba, M. Heavy metal distribution between contaminated soil and Paulownia tomentosa, in a pilot-scale assisted phytoremediation study: Influence of different complexing agents. Chemosphere 2008, 72, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Mokany, K.; Raison, R.J.; Prokushkin, A.S. Critical analysis of root: Shoot ratios in terrestrial biomes. Global Change Biol. 2006, 12, 84–96. [Google Scholar] [CrossRef]
- Sebastiani, L.; Scebba, F.; Tognetti, R. Heavy metal accumulation and growth response in poplar clones Eridano (Populus deltoides × maximowiczii) and I-214 (P. × euramericana) exposed to industrial waste. Environ. Exp. Bot. 2004, 52, 79–88. [Google Scholar] [CrossRef]
- Reichman, S.M. The Responses of Plants to Metal Toxicity: A Review Focusing on Copper, Manganese & Zinc; Australian Minerals & Energy Environment Foundation: Melbourne, Australia, 2002. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Xiang, W.; Ma, Y.; Lei, P.; Tian, D.; Deng, X.; Yan, W.; Fang, X. Growth and Heavy Metal Accumulation of Koelreuteria Paniculata Seedlings and Their Potential for Restoring Manganese Mine Wastelands in Hunan, China. Int. J. Environ. Res. Public Health 2015, 12, 1726-1744. https://doi.org/10.3390/ijerph120201726
Huang Z, Xiang W, Ma Y, Lei P, Tian D, Deng X, Yan W, Fang X. Growth and Heavy Metal Accumulation of Koelreuteria Paniculata Seedlings and Their Potential for Restoring Manganese Mine Wastelands in Hunan, China. International Journal of Environmental Research and Public Health. 2015; 12(2):1726-1744. https://doi.org/10.3390/ijerph120201726
Chicago/Turabian StyleHuang, Zhihong, Wenhua Xiang, Yu'e Ma, Pifeng Lei, Dalun Tian, Xiangwen Deng, Wende Yan, and Xi Fang. 2015. "Growth and Heavy Metal Accumulation of Koelreuteria Paniculata Seedlings and Their Potential for Restoring Manganese Mine Wastelands in Hunan, China" International Journal of Environmental Research and Public Health 12, no. 2: 1726-1744. https://doi.org/10.3390/ijerph120201726






