Exposure to the Riot Control Agent CS and Potential Health Effects: A Systematic Review of the Evidence
Abstract
:1. Introduction
2. Experimental Section
Methods
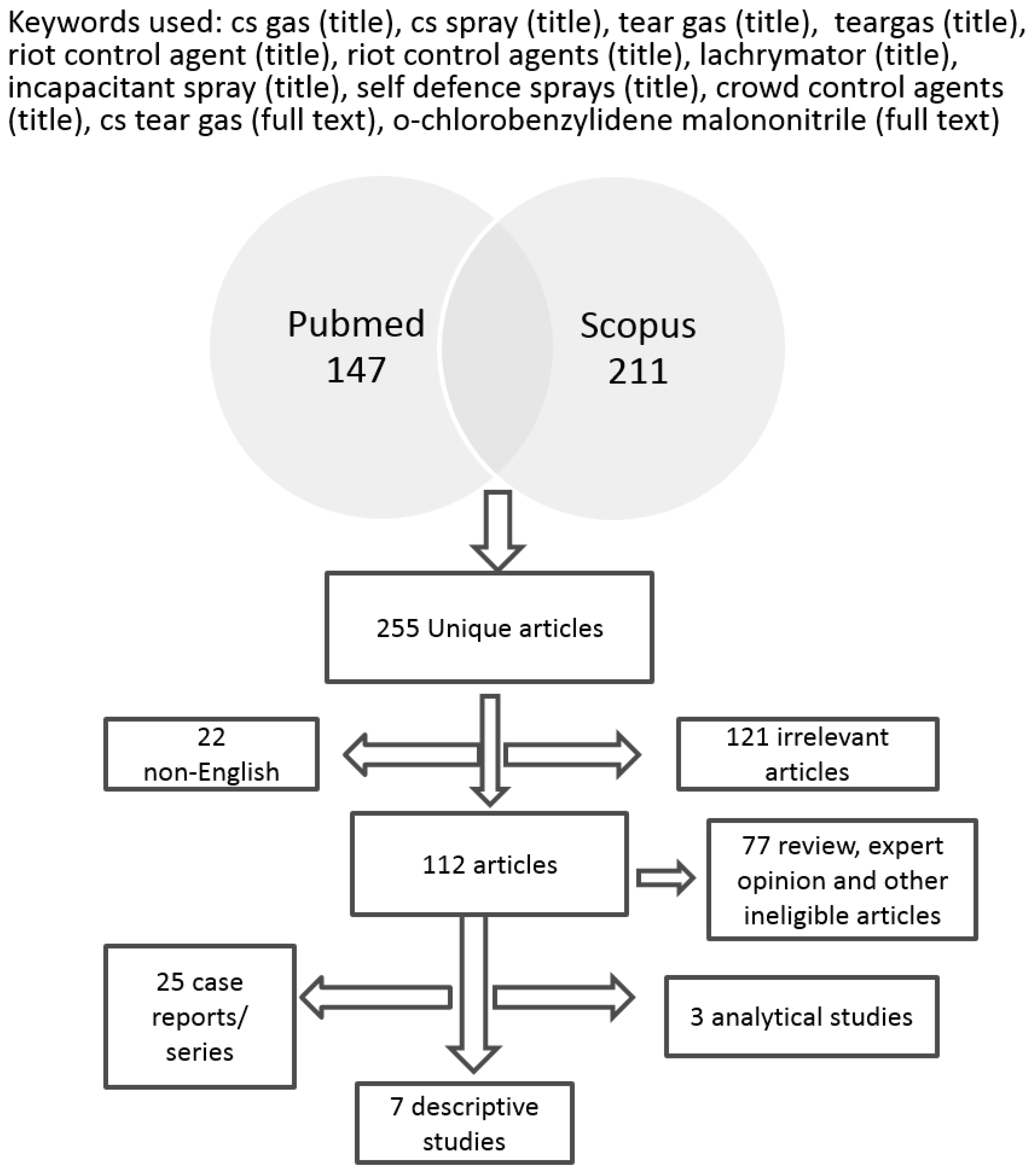
3. Results
| Year | Author | n | Country | Reference |
|---|---|---|---|---|
| 2012 | Bhargava et al. | 1 | UK | [14] |
| 2011 | Shamphu et al. | 1 | UK | [15] |
| 2011 | Wu et al. | 1 | UK | [16] |
| 2010 | Kain et al. | 1 | UK | [17] |
| 2009 | Agrawal et al. | 1 | UK | [5] |
| 2009 | Karaman et al. | 1 | TURKEY | [18] |
| 2006 | Hardwicke et al. | 1 | UK | [19] |
| 2005 | Horton et al. | 7 | USA | [20] |
| 2005 | Watson et al. | 7 | UK | [21] |
| 2005 | Morrone et al. | 1 | ITALY | [22] |
| 2004 | Davey et al. | 3 | UK | [23] |
| 2003 | Solomon et al. | 7 | ISRAEL | [24] |
| 2003 | Horton et al. | 5 | USA | [25] |
| 2001 | Southward et al. | 1 | UK | [13] |
| 2001 | Varma et al. | 1 | UK | [26] |
| 2000 | Barlow | 1 | UK | [27] |
| 2000 | Hill et al. | 1 | USA | [28] |
| 1999 | Sommer et al. | 1 | UK | [29] |
| 1998 | Breakell et al. | 23 | UK | [30] |
| 1997 | Kiel et al. | 6 | UK | [31] |
| 1996 | Roth et al. | 1 | USA | [32] |
| 1993 | Bhattacharya et al. | 2 | UK | [33] |
| 1993 | Parneix-Spake et al. | 11 | FRANCE | [34] |
| 1992 | Hu et al. | 1 | USA | [35] |
| 1991 | Ro et al. | 2 | SOUTH KOREA | [36] |
| Year | Author | No of Cases | Study Type | Country | Reference |
|---|---|---|---|---|---|
| 2014 | Hout et al. | 5298 | Analytical | USA | [37] |
| 2014 | Hout et al. | 6723 | Analytical | USA | [38] |
| 2007 | Hankin et al. | 21 | Descriptive | UK | [39] |
| 2004 | Euripidou et al. | 152 | Descriptive | UK | [40] |
| 2003 | Nathan et al. | 30 | Descriptive | UK | [41] |
| 2003 | Karagama et al. | 34 | Analytical | UK | [42] |
| 2002 | Thomas et al. | 38 | Descriptive | USA | [43] |
| 1998 | Wheeler et al. | 597 | Descriptive | UK | [7] |
| 1996 | Anderson et al. | 184 | Descriptive | HONG KONG | [44] |
| 1995 | Zekri et al. | 96 | Descriptive | HONG KONG | [45] |
3.1. Case Reports/Case Series
3.2. Conditions of Exposure to CS
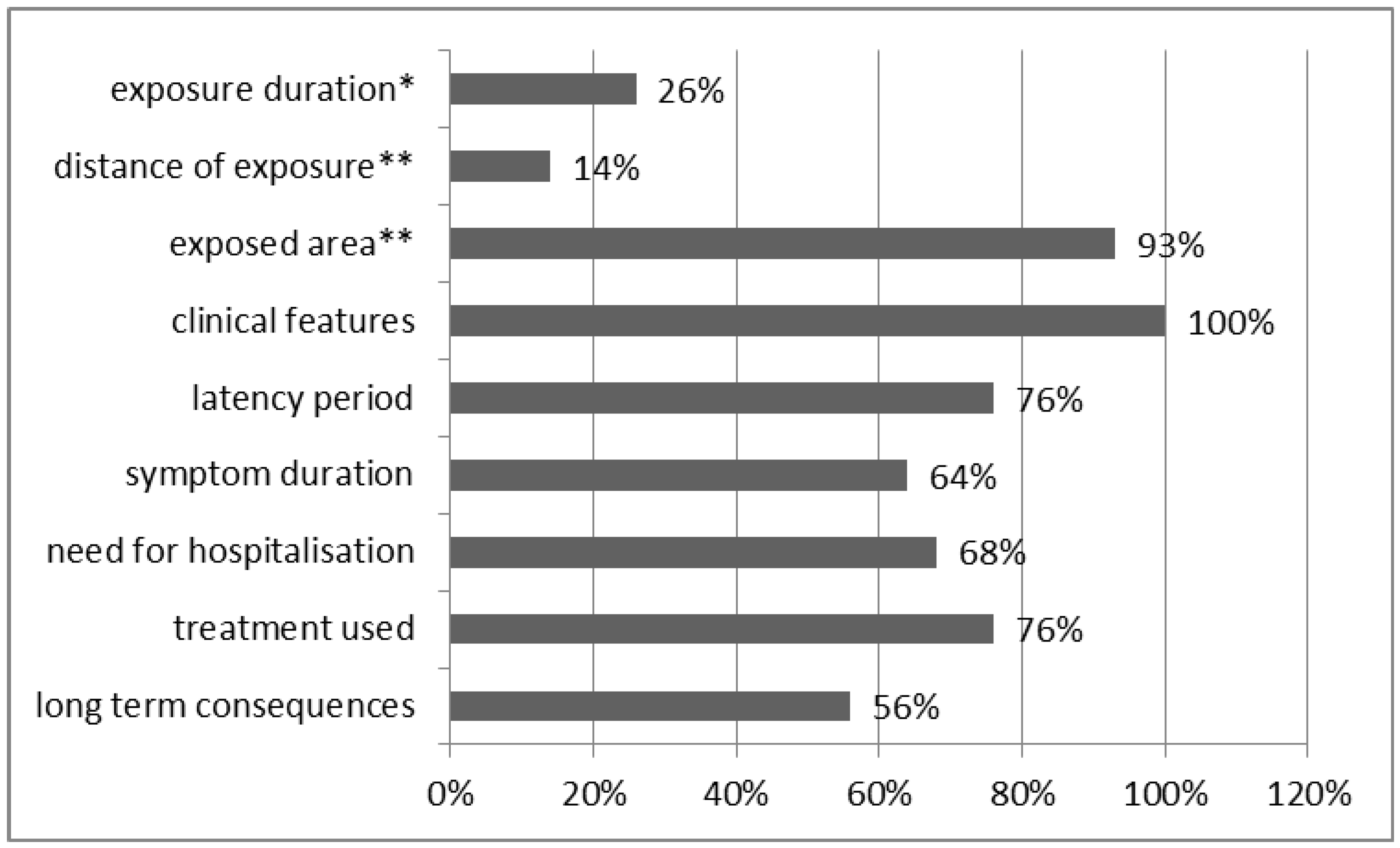
3.3. Clinical Effects of Exposure to CS
3.4. Dermal Clinical Effects
| Effect | Expected Latency Period |
|---|---|
| Blistering rash/bullae [5,13,16,21,29,34,36] | 12 h to a week [5,13,16,21,29,34,36] |
| Erythema/redness [5,16,19,21,22,24,26,28,34,36] | some minutes to 4 days [5,19,21,24,26,28,34,36] |
| Oedema/swelling [21,24,26,28,34] | 1 h to 3 days [21,24,26,28,34] |
| Burning-sensation [21,22,23,25,33,35,36] | Immediate [21,23,33,35,36] |
| Burns [5,13,17,19] | >2 days [5,13,19] |
| Pruritus [21,22,28] | within some days [21,28] |
| Eczema, seborrhoeic dermatitis [21,36] | 4 h to some days [21,36] |
| Acute generalised exanthematosus pistulitis/skin rash [16,28] | 1–2 weeks [16,28] |
| Allergic contact dermatitis [14,15,21,29,36] | Within a week [14,15,21,29,36] |
| Dermal irritation/pain [5,16,20,21,26,29] | >24 h [5,20,21,26,29] |
3.5. Respiratory Clinical Effects
| Effect | Expected Latency Period |
|---|---|
| Cough [18,23,28,32,35] | Immediate to 2 days [18,23,28,32,35] |
| Dyspnoea,chest tightness [21,27,28,30,35] | Immediate/within minutes [21,27,28,30,35] |
| Respiratory irritation [20,24,25] | Within minutes [24] |
| Laryngeal obstruction [18] | About 3 weeks [18] |
| Hypersensitivity reaction with pneumonitis and bronchocostriction/ RADS [28,32,35] | 1–2 weeks [28] |
| Laryngospasm [23] | 12 hours after exposure, during anaisthesia [23] |
| Sore throat/burning of the throat [24,25,32,33,35] | Immediate/within minutes [24,32,33,35] |
3.6. Ocular Clinical Effects
| Effect | Expected Latency Period |
|---|---|
| Lacrymation [18,21,23,24,27,33] | immediate [18,21,23,24,27,33] |
| Blinkng/ blepharospasm [18,33] | immediate [18,33] |
| Sting of the eyes [21,23,33] | Immediate [21,23,33] |
| Eye irritation [20,24,25,30,33,35] | immediate/within minutes [20,24,30,33,35] |
| Reduced vision [5] | >24 h [5] |
| Conjuctivitis [16,18,21,24,28,31,33] | some minutes [18,21] |
3.7. Gastrointestinal Clinical Effects
3.8. Multisystem Hypersensitivity Reaction
3.9. Long Term and Life Threatening Effects
3.10. Complications during Anaesthesia and Exposure to CS
3.11. Descriptive Studies
3.12. Analytical Studies
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Carron, P.N.; Yersin, B. Management of the effects of exposure to tear gas. BMJ 2009, 19. [Google Scholar] [CrossRef]
- Schep, L.J.; Slaughter, R.J.; McBride, D.I. Control agents: The tear gases CN, CS and OC—A medical review. Riot. J. R Army Med. Corps. 2013. [Google Scholar] [CrossRef]
- Salem, H.; Gutting, B.; Kluchinsky, T.; Boardman, C.; Tuorinsky, S.; Hout, J. Riot control agents. In Medical Aspects of Chemical Warfare; US Army Medical Department: Washington, DC, USA, 2008; Chapter 13. [Google Scholar]
- Stark, M.M.; Knight, M. “Safety” of chemical batons. Lancet 1998, 352. [Google Scholar] [CrossRef]
- Agrawal, Y.; Thornton, D.; Phipps, A. CS gas—Completely safe? A burn case report and literature review. Burns 2009, 35, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Fine, J.; Epstein, P.; Kelsey, K.; Reynolds, P.; Walker, B. Tear gas—Harassing agent or toxic chemical weapon? JAMA 1989, 262, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, H.; MacLehose, R.; Euripidou, F.; Murray, V. Surveillance into crowd-control agents. Lancet 1998, 352, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Olajos, E.J.; Salem, H. Riot control agents: Pharmacology, toxicology, biochemistry and chemistry. J. Appl. Toxicol. 2001, 21, 355–391. [Google Scholar] [CrossRef] [PubMed]
- Von Däniken, A.; Friederich, U.; Lutz, W.K.; Schlatter, C. Tests for mutagenicity in Salmonella and covalent binding to DNA and protein in the rat of the riot control agent o-chlorobenzylidene malononitrile (CS). Arch. Toxicol. 1981, 49, 15–27. [Google Scholar]
- Brown, A.; Cattanach, P.; Edwards, I.; McBride, D.; Caspary, W.J. Responses of the L5178Y tk+/tk-Mouse lymphoma cell forward mutation assay. II: 18 coded chemicals. Environ. Mol. Mutagen. 1988, 11, 91–118. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, E.C.; Delbressine, L.P.; Waegemaekers, T.H.; Seutter-Berlage, F. 2-Chlorobenzylmercapturic acid, a metabolite of the riot control agent 2-chlorobenzylidene malononitrile (CS) in the rat. Arch. Toxicol. 1983, 54, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Greaves, I. The use of chemical incapacitant sprays: A review. J. Trauma 2002, 52, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Southward, R.D. Cutaneous burns from CS incapacitant spray. Med. Sci. Law 2001, 41, 74–77. [Google Scholar] [PubMed]
- Bhargava, K.; Baneriee, P.; White, I.R. Investigating contact allergy to CS spray. Contact Dermatitis. 2012, 66, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Shamphu, S.; Kurtis, R. Allergic contact dermatitis due to CS spray. Emerg. Med. J. 2011, 28. [Google Scholar] [CrossRef]
- Wu, K.; Husain, A.; Barry, R. Acute generalized exanthematous pustulosis induced by a topical agent: 2-chlorobenzylidene malonitrile (CS) gas. Br. J. Dermatol. 2011, 164, 227–278. [Google Scholar] [CrossRef] [PubMed]
- Kain, N.; Mishra, A.; James, M.I. Guidance needed on secondary effects of CS gas on staff. BMJ 2010. [Google Scholar] [CrossRef]
- Karaman, E.; Erturan, S.; Duman, C.; Yaman, M.; Duman, G.U. Acute laryngeal and bronchial obstruction after CS (o-chlorobenzylidenemalononitrile) gas inhalation. Eur. Arch. Otorhinolaryngol. 2009, 266, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Hardwicke, J.; Satti, U. Facial burns after exposure to CS spray. Injury Extra. 2006, 37, 133–134. [Google Scholar] [CrossRef]
- Horton, D.K.; Burgess, P.; Rossiter, S.; Kaye, W.E. Secondary contamination of emergency department personnel from o-chlorobenzylidene malononitrile exposure, 2002. Ann. Emerg. Med. 2005, 45, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Rycroft, R. Unintended cutaneous reactions to CS spray. Contact Dermatitis. 2005, 53, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Morrone, A.; Sacerdoti, G.; Franco, G.; Corretti, R.; Fazio, M. Tear gas dermatitis. Clin. Exp. Dermatol. 2005, 30, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Davey, A.; Moppett, I.K. Postoperative complications after CS spray exposure. Anaesthesia 2004, 59, 1219–1220. [Google Scholar] [CrossRef] [PubMed]
- Solomon, I.; Kochba, I.; Eizenkraft, E.; Maharshak, N. Report of accidental CS ingestion among seven patients in central Israel and review of the current literature. Arch. Toxicol. 2003, 77, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.K.; Berkowitz, Z.; Kaye, W.E. Secondary contamination of ED personnel from hazardous materials events, 1995–2001. Am. J. Emerg. Med. 2003, 21, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Holt, P.J. Severe cutaneous reaction to CS gas. Clin. Exp. Dermatol. 2001, 26, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Barlow, N. Precautions should be taken before anaesthetising anybody, who has been exposed to CS gas to prevent complications such as this making intubation impossible. Resuscitation 2000, 47. [Google Scholar] [CrossRef]
- Hill, A.R.; Silverberg, N.B.; Mayorga, D.; Baldwin, H.E. Medical hazards of the tear gas CS. A case of persistent, multisystem, hypersensitivity reaction and review of the literature. Medicine (Baltimore) 2000, 79, 234–240. [Google Scholar] [CrossRef]
- Sommer, S.; Wilkinson, S.M. Exposure-pattern dermatitis due to CS gas. Contact Dermatitis. 1999, 40, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Breakell, A.; Bodiwala, G.G. CS gas exposure in a crowded night club: the consequences for an accident and emergency department. J. Accid. Emerg. Med. 1998, 15, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Kiel, A.W. Ocular exposure to CS gas: The importance of correct early management. Eye (Lond.) 1997, 11, 759–760. [Google Scholar] [CrossRef]
- Roth, V.S.; Franzblau, A. RADS after exposure to a riot-control agent: A case report. J. Occup. Environ. Med. 1996, 38, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.T.; Hayward, A.W. CS gas—Implications for the anaestetist. Anaesthesia 1993, 48, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Parneix-Spake, A.; Theisen, A.; Rouzeau, J.C.; Revuz, J. Severe cutaneous reactions to self-defense sprays. Arch. Dermatol. 1993, 129. [Google Scholar] [CrossRef]
- Hu, H.; Christiani, D. Reactive airways dysfunction after exposure to teargas. Lancet. 1992, 339. [Google Scholar] [CrossRef] [PubMed]
- Ro, Y.S.; Lee, C.W. Tear gas dermatitis. Allergic contact sensitization due to CS. Int. J. Dermatol. 1991, 30, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Hout, J.J.; White, D.W.; Stevens, M.; Stubner, A.; Arino, A.; Knapik, J. Evaluation of an intervention to reduce tear gas exposures and associated acute respiratory illnesses in a US Army Basic Combat Training cohort. Open Epidemiol. J. 2014, 7, 37–45. [Google Scholar]
- Hout, J.J.; White, D.W.; Artino, A.R.; Knapik, J.J. o-chlorobenzylidene malononitrile (CS riot control agent) associated acute respiratory illnesses in a U.S. Army Basic Combat Training cohort. Mil Med. 2014, 179, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Hankin, S.M.; Ramsay, C.N. Investigation of accidental secondary exposure to CS agent. Clin. Toxicol. (Phila.) 2007, 45, 409–411. [Google Scholar] [CrossRef]
- Euripidou, E.; MacLehose, R.; Fletcher, A. An investigation into the short term and medium term health impacts of personal incapacitant sprays. A follow up of patients reported to the National Poisons Information Service (London). Emerg. Med. J. 2004, 21, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Wood, H.; Rix, K.; Wright, E. Long-term psychiatric morbidity in the aftermath of CS spray trauma. Med. Sci. Law 2003, 43, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Karagama, Y.G.; Newton, J.R.; Newbegin, C.J. Short-term and long-term physical effects of exposure to CS spray. J. R. Soc. Med. 2003, 96, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Smith, P.A.; Rascona, D.A.; Louthan, J.D.; Gumpert, B. Acute pulmonary effects from o-chlorobenzylidenemalonitrile “tear gas”: A unique exposure outcome unmasked by strenuous exercise after a military training event. Mil. Med. 2002, 167, 136–139. [Google Scholar] [PubMed]
- Anderson, P.J.; Lau, G.S.; Taylor, W.R.; Critchley, J.A. Acute effects of the potent lacrimator o-chlorobenzylidene malononitrile (CS) tear gas. Hum. Exp. Toxicol. 1996, 15, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Zekri, A.M.; King, W.W.; Yeung, R.; Taylor, W.R. Acute mass burns caused by o-chlorobenzylidene malononitrile (CS) tear gas. Burns 1995, 21, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Arbak, P.; Elknur BaGer, I.; Kumbasar, Ö.O.; Ülger, F.; Zeki Kiliçaslan, Z.; Evyapan, F. Long term effects of tear gases on respiratory system: Analysis of 93 cases. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Payne-James, J.J.; Smith, G.; Rivers, E.; O’Rourke, S.; Stark, M.; Sutcliffe, N. Effects of incapacitant spray deployed in the restraint and arrest of detainees in the Metropolitan Police Service area, London, UK: A prospective study. Forensic Sci. Med. Pathol. 2014, 10, 62–68. [Google Scholar] [CrossRef] [PubMed]
- CDC. Chemical Emergencies. Case Definition. Riot Control Agents. Available online: http://emergency.cdc.gov/agent/riotcontrol/casedefinition.asp (accessed on 21 January 2015).
- Riches, J.R.; Read, R.W.; Black, R.M.; Harrison, J.M.; Shand, D.A.; Tomsett, E.V.; Newsome, C.R.; Bailey, N.C.; Roughley, N.; Gravett, M.R.; et al. The development of an analytical method for urinary metabolites of the riot control agent 2-chlorobenzylidene malononitrile (CS). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 928, 125–130. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitroglou, Y.; Rachiotis, G.; Hadjichristodoulou, C. Exposure to the Riot Control Agent CS and Potential Health Effects: A Systematic Review of the Evidence. Int. J. Environ. Res. Public Health 2015, 12, 1397-1411. https://doi.org/10.3390/ijerph120201397
Dimitroglou Y, Rachiotis G, Hadjichristodoulou C. Exposure to the Riot Control Agent CS and Potential Health Effects: A Systematic Review of the Evidence. International Journal of Environmental Research and Public Health. 2015; 12(2):1397-1411. https://doi.org/10.3390/ijerph120201397
Chicago/Turabian StyleDimitroglou, Yiannis, George Rachiotis, and Christos Hadjichristodoulou. 2015. "Exposure to the Riot Control Agent CS and Potential Health Effects: A Systematic Review of the Evidence" International Journal of Environmental Research and Public Health 12, no. 2: 1397-1411. https://doi.org/10.3390/ijerph120201397







