Sex-Dependent Depression-Like Behavior Induced by Respiratory Administration of Aluminum Oxide Nanoparticles
Abstract
:1. Introduction
2. Methods
2.1. Nanomaterial and Animals
2.2. Animal Treatment
2.3. Open-Field Tests
2.4. Forced Swimming Test
2.5. RNA Isolation and Quantitative Real-Time PCR Assay
2.6. Pathological Analysis
2.7. Aluminum Burden
2.8. Metabolomics Analysis
2.9. Data Analysis
3. Results
3.1. Female Mice Demonstrated Depressive-Like Behavior Following Al2O3 NP Exposure.
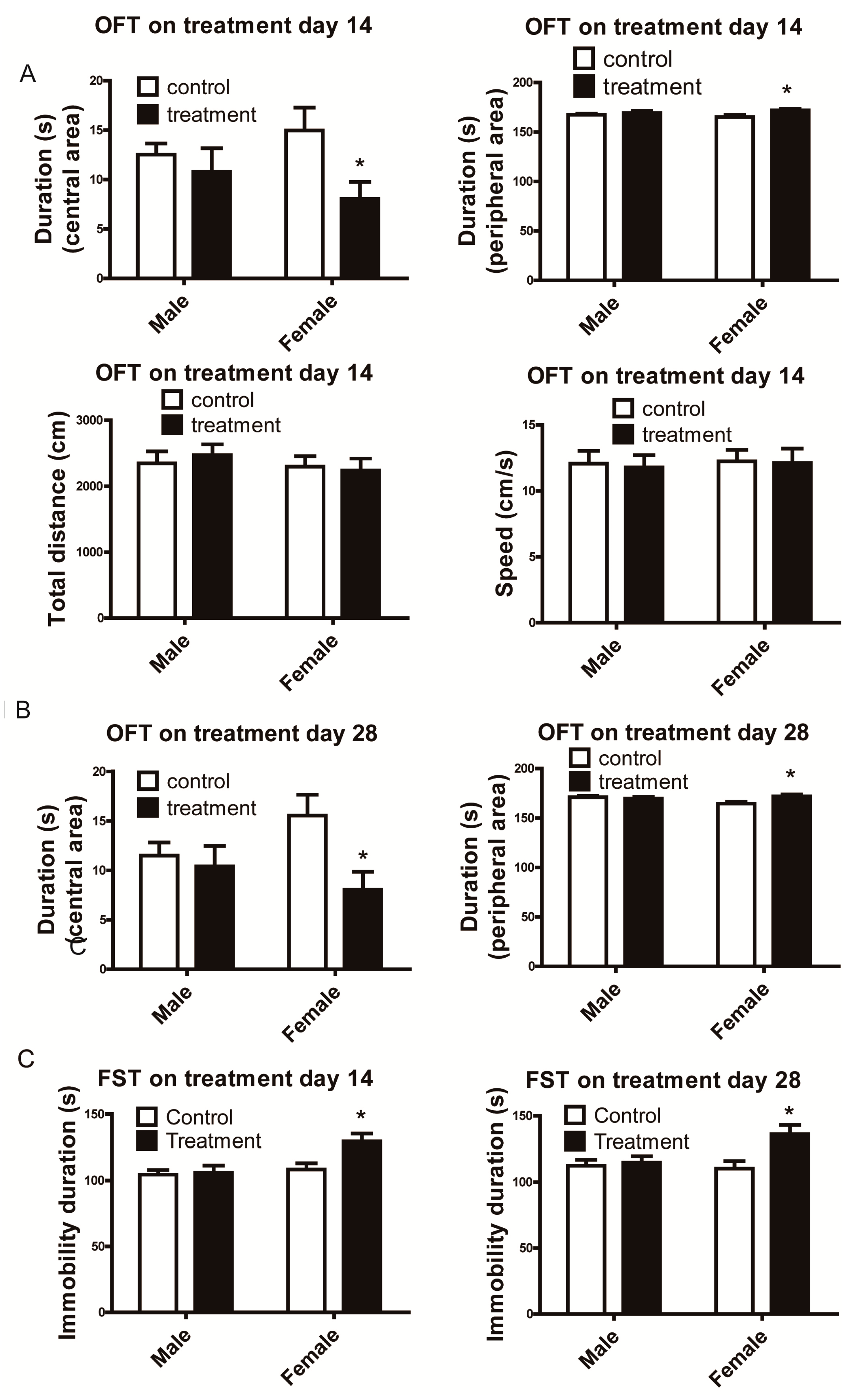
3.2. Aluminum Burden Varied in Brain and Lung Tissues of Mice

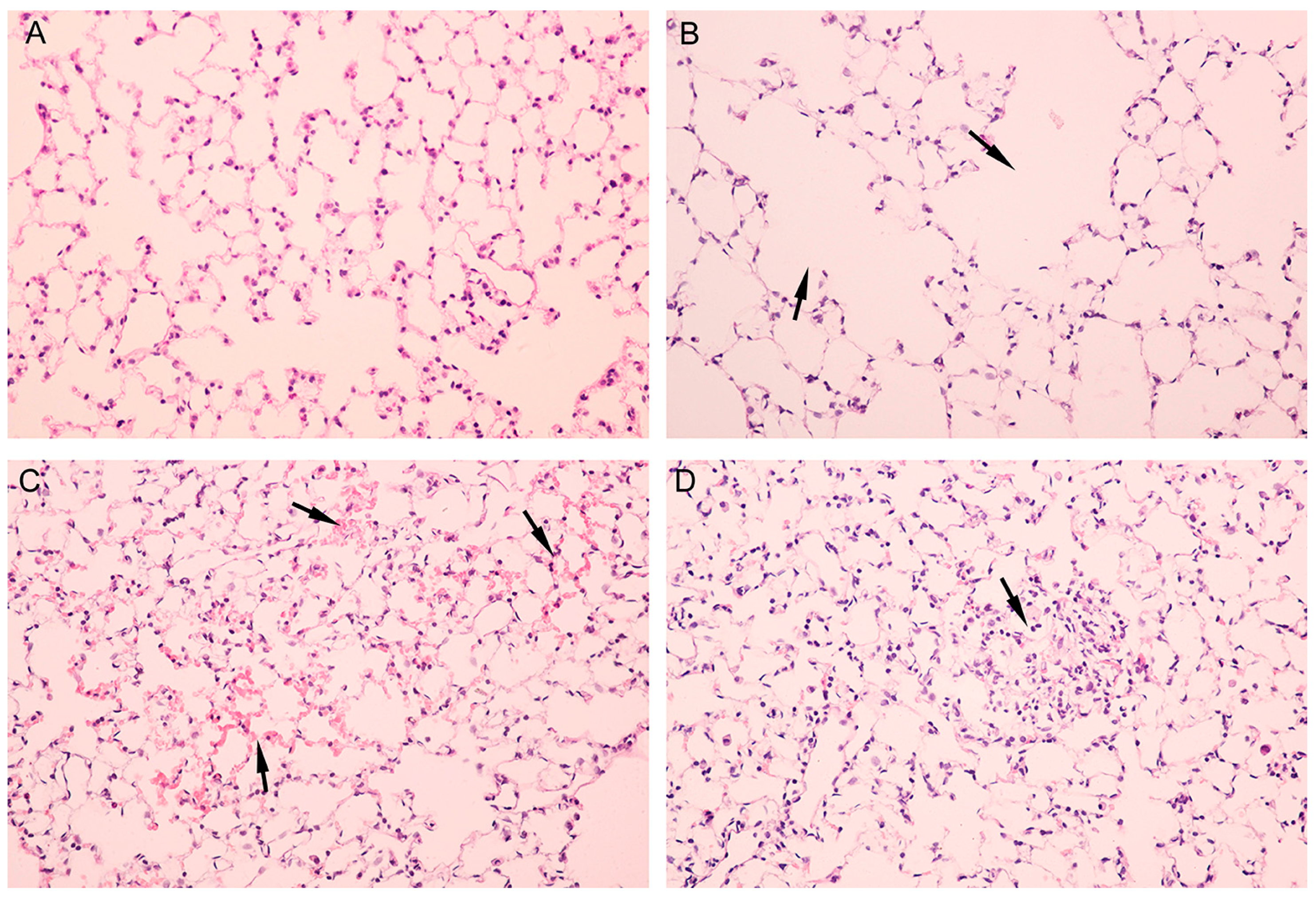
3.3. Pathological Alterations of Lung but not Brain Tissues of Mice Were Observed
| Group | Lesion Severity Grade | Average Severity Grade | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| male control | 4 | 1 | |||
| female control | 4 | 1 | |||
| male nano-Al2O3 | 3 | 1 | 2.25 ± 0.50 * | ||
| female nano-Al2O3 | 2 | 2 | 2.5 ± 0.58 * | ||
3.4. Expressions of Mental Disorder Involved Genes Were Modulated in a Sex-Dependent Manner
3.5. Metabolomics Analysis Explored the Potential Role of Glutamate
| Control | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | F Value | p Value | |||||
| Fold Change | Fold Change | Fold Change | Fold Change | Treatment | Sex | Interaction | Treatment | Sex | Interaction | |
| SNAP29 | 1.03 ± 0.10 | 1.02 ± 0.12 | 0.95 ± 0.11 | 0.62±0.14 *,# | 24.64 | 12.36 | 10.95 & | <0.0001 | 0.0022 | 0.0035 |
| UFD1L | 1.01 ± 0.07 | 1.02 ± 0.06 | 0.61 ± 0.08 * | 0.65±0.08 * | 167.00 | 0.7042 | 0.2535 | <0.0001 | 0.4113 | 0.6201 |
| ZDHHC8 | 1.04 ± 0.05 | 1.05 ± 0.07 | 0.95 ± 0.07 * | 0.58±0.06 *,# | 131.40 | 41.09 | 46.23 & | <0.0001 | <0.0001 | <0.0001 |
| PDLIM5 | 1.01 ± 0.08 | 1.05 ± 0.10 | 0.71 ± 0.10 * | 0.82±0.14 * | 36.64 | 2.935 | 0.639 | <0.0001 | 0.1022 | 0.4334 |
| HSPA1A | 1.06 ± 0.10 | 0.99 ± 0.07 | 0.54 ± 0.11 * | 0.56±0.09 * | 154.30 | 0.4273 | 1.385 | <0.0001 | 0.5207 | 0.2531 |
| PTEN | 0.99 ± 0.08 | 1.03 ± 0.09 | 0.63 ± 0.11 * | 0.67±0.12 * | 75.86 | 0.94 | 0.00 | <0.0001 | 0.3447 | 1.0000 |
| GDNF | 1.01 ± 0.07 | 0.98 ± 0.05 | 0.65 ± 0.06 * | 0.32±0.14 *,# | 204.00 | 25.41 | 17.65 & | <0.0001 | <0.0001 | 0.0004 |
| SOD2 | 0.97 ± 0.06 | 0.99 ± 0.08 | 0.67 ± 0.12 * | 0.56±0.07 * | 109.10 | 1.66 | 3.46 | <0.0001 | 0.2125 | 0.0776 |
| GNAS | 1.01 ± 0.09 | 1.06 ± 0.12 | 0.88 ± 0.15 | 0.70±0.14 *,# | 22.30 | 1.57 | 4.91 & | 0.0001 | 0.2247 | 0.0384 |
| NOS3 | 0.96 ± 0.11 | 1.00 ± 0.04 | 0.71 ± 0.08 * | 0.93±0.15 # | 14.42 | 9.52 | 4.56 & | 0.0011 | 0.0058 | 0.0452 |
| DLST | 1.01 ± 0.04 | 0.97 ± 0.06 | 0.91 ± 0.12 | 0.87±0.06 | 10.34 | 1.66 | 0.00 | 0.0043 | 0.2130 | 1.0000 |
| GRIN2A | 0.97 ± 0.08 | 1.01 ± 0.06 | 1.10 ± 0.18 | 2.78±0.17 *,# | 423.70 | 317.70 | 288.20 & | <0.0001 | <0.0001 | <0.0001 |
| DGKH | 1.01 ± 0.04 | 1.06 ± 0.09 | 2.14 ± 0.09 * | 0.72±0.06 *,# | 175.00 | 526.21 | 605.94 & | <0.0001 | <0.0001 | <0.0001 |
| MT2A | 1.01 ± 0.07 | 1.02 ± 0.15 | 1.98 ± 0.07 * | 2.34±0.13 *,# | 639.50 | 16.70 | 14.94 & | 0.0010 | 0.0006 | <0.0001 |
| CACNA1G | 1.04 ± 0.07 | 1.03 ± 0.06 | 2.77 ± 0.13 * | 0.75±0.12 *,# | 516.75 | 938.68 | 887.50 & | <0.0001 | <0.0001 | <0.0001 |
| KCNQ | 1.00 ± 0.04 | 0.97 ± 0.08 | 2.86 ± 0.10 * | 0.72±0.11 *,# | 317.03 | 621.19 | 609.14 & | <0.0001 | <0.0001 | <0.0001 |
| Metabolite | HMDB ID | Fold Change | Male Al2O3 NPs vs. Male Control | Female Al2O3 NPs vs. Male Control | |||||
|---|---|---|---|---|---|---|---|---|---|
| Male Al2O3 NPs | Female Control | Female Al2O3 NPs | F Value | p Value | F Value | p Value | |||
| L-Serine | HMDB00187 | 1.98 | 1.03 | 4.26 # | 13.63 | 0.002 | 53.54 | <0.0001 | |
| Dihydroxy acetone Phosphate | HMDB01473 | - | 0.83 | 2.97 # | 0.43 | 0.5261 | 14.9 | 0.0017 | |
| Inosine | HMDB00195 | 2.05 | 1.14 | 2.61 | 12.68 | 0.0022 | 13.01 | 0.0019 | |
| Prostaglandin E2 | HMDB01220 | 2.43 | 1.27 | 4.02 | 22.31 | 0.0001 | 49.69 | <0.0001 | |
| Acetyl-L-Carnitine | HMDB00201 | 2.37 | 0.96 | 3.86 | 28.74 | <0.0001 | 37.21 | <0.0001 | |
| Pyroglutamic Acid | HMDB00267 | 2.53 | 0.89 | 0.52 # | 32.57 | <0.0001 | 24.52 | <0.0001 | |
| Riboflavin | HMDB00244 | 3.83 | 1.07 | 2.18 | 36.53 | <0.0001 | 27.73 | <0.0001 | |
| Inosine 5-Monophosphate | HMDB00175 | 1.89 | 0.93 | 1.68 | 11.42 | 0.0026 | 9.52 | 0.0058 | |
| l-Glutamic Acid | HMDB00148 | - | 1.08 | 0.17 # | 1.69 | 0.2125 | 103.89 | <0.0001 | |
| 7b-Hydroxycholesterol | HMDB06119 | 0.48 | 0.77 | - | 28.49 | <0.0001 | 0.89 | 0.4213 | |
| 2-Hydroxyestrone | HMDB00343 | - | 0.98 | 0.48 | 0.25 | 0.6178 | 41.25 | <0.0001 | |
| Dl-Glyceraldehyde | HMDB01051 | 0.3 | 1.06 | 0.46 | 59.77 | <0.0001 | 38.56 | <0.0001 | |
| Petroselinic Acid | HMDB02080 | 0.21 | 1.18 | 0.48 | 75.86 | <0.0001 | 42.59 | <0.0001 | |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nogueira, J.B. Air pollution and cardiovascular disease. Rev. Port. Cardiol. 2009, 28, 715–733. [Google Scholar] [PubMed]
- Corbin, J.C. PM0.1 particles from aircraft may increase risk of vascular disease. BMJ 2013, 347. [Google Scholar] [CrossRef] [PubMed]
- Gavett, S.H.; Haykal-Coates, N.; Copeland, L.B.; Heinrich, J.; Gilmour, M.I. Metal composition of ambient PM2.5 influences severity of allergic airways disease in mice. Environ. Health Perspect. 2003, 111, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Veranth, J.M.; Kaser, E.G.; Veranth, M.M.; Koch, M.; Yost, G.S. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part. Fiber Toxicol. 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Reff, A.; Bhave, P.V.; Simon, H.; Pace, T.G.; Pouliot, G.A.; Mobley, J.D.; Houyoux, M. Emissions inventory of PM2.5 trace elements across the United States. Environ. Sci. Technol. 2009, 43, 5790–5796. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Donaldson, K.; Hadoke, P.W.; Boon, N.A.; MacNee, W.; Cassee, F.R.; Sandstrom, T.; Blomberg, A.; Newby, D.E. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Zheng, H.; Zhang, Z.R.; Li, M.; Huang, Z.Y.; Schluesener, H.J.; Li, Y.Y.; Xu, S.Q. Glia activation induced by peripheral administration of aluminum oxide nanoparticles in rat brains. Nanomedicine 2009, 5, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Korszun, A. Sex, trauma, stress hormones and depression. Mol. Psychiatry 2010, 15, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Galea, L.A.; McEwen, B.S.; Tanapat, P.; Deak, T.; Spencer, R.L.; Dhabhar, F. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 1997, 81, 689–697. [Google Scholar] [CrossRef]
- Yokota, S.; Takashima, H.; Ohta, R.; Saito, Y.; Miyahara, T.; Yoshida, Y.; Negura, T.; Senuma, M.; Usumi, K.; Hirabayashi, N.; et al. Nasal instillation of nanoparticle-rich diesel exhaust particles slightly affects emotional behavior and learning capability in rats. J. Toxicol. Sci. 2011, 36, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Oshio, S.; Iwata, M.; Saburi, H.; Odagiri, T.; Udagawa, T.; Sugawara, I.; Umezawa, M.; Takeda, K. In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Part. Fibre Toxicol. 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.K.; Kovalycsik, T.; Sun, Q.; Rajagopalan, S.; Nelson, R.J. Combined effects of exposure to dim light at night and fine particulate matter on C3H/HeNHsd mice. Behav. Brain Res. 2015, 294, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Y.; Zhang, T.; Ren, G.; Yang, Z. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J. Biomed. Sci. 2012, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, D.A.; Bortolato, M.; Godar, S.C.; Sander, T.K.; Iwata, N.; Pakbin, P.; Shih, J.C.; Berhane, K.; McConnell, R.; Sioutas, C.; Finch, C.E.; Morgan, T.E. Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depression-like responses. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Liu, X.; Pelkowski, S.; Palmer, B.; Conrad, K.; Oberdorster, G.; Weston, D.; Mayer-Proschel, M.; Cory-Slechta, D.A. Early postnatal exposure to ultrafine particulate matter air pollution: Persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ. Health Perspect. 2014, 122, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Price, J.L.; Simpson, J.R., Jr.; Todd, R.D.; Reich, T.; Vannier, M.; Raichle, M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997, 386, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I. Neuroimaging studies of mood disorder effects on the brain. Biol. Psychiatry 2003, 54, 338–352. [Google Scholar] [CrossRef]
- Banasr, M.; Duman, R.S. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol. Disord. Drug Targets 2007, 6, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Choudary, P.V.; Molnar, M.; Evans, S.J.; Tomita, H.; Li, J.Z.; Vawter, M.P.; Myers, R.M.; Bunney, W.E., Jr.; Akil, H.; Watson, S.J.; Jones, E.G. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc. Natl. Acad. Sci. USA 2005, 102, 15653–15658. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Winter, C.; Coquery, N.; Heinz, A.; Morgenstern, R.; Kupsch, A.; Juckel, G. Lesion of the medial prefrontal cortex and the subthalamic nucleus selectively affect depression-like behavior in rats. Behav. Brain Res. 2010, 213, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Niimi, K.; Itakura, C. Emotional behavior in heterozygous rolling mouse Nagoya Ca v 2.1 channel mutant mice. Neurobiol. Aging 2011, 32, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Szapiel, S.V.; Elson, N.A.; Fulmer, J.D.; Hunninghake, G.W.; Crystal, R.G. Bleomycin-induced interstitial pulmonary diseases in the nude, athymic mouse. Am. Rev. Respir. Dis. 1979, 120, 893–899. [Google Scholar] [PubMed]
- Xu, B.; Chen, M.; Ji, X.; Mao, Z.; Zhang, X.; Wang, X.; Xia, Y. Metabolomic profiles delineate the potential role of glycine in gold nanorod-induced disruption of mitochondria and blood-testis barrier factors in TM-4 cells. Nanoscale 2014, 6, 8265–8273. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, X.; Zhou, Z.; Lei, Y.; Ma, M.; Cao, R.; Sun, T.; Xu, J.; Huo, M.; Cao, R.; Wen, C.; Che, Y. Prenatal exposure to nanoparticulate titanium dioxide enhances depressive-like behaviors in adult rats. Chemosphere 2014, 96, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zwingman, T.A.; Fletcher, C.F. In vivo analysis of voltage-dependent calcium channels. J. Bioenerg. Biomembr. 2003, 35, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Whalley, H.C.; Papmeyer, M.; Romaniuk, L.; Johnstone, E.C.; Hall, J.; Lawrie, S.M.; Sussmann, J.E.; McIntosh, A.M. Effect of variation in diacylglycerol kinase eta (DGKH) gene on brain function in a cohort at familial risk of bipolar disorder. Neuropsychopharmacology 2012, 37, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Kittel-Schneider, S.; Gessner, A.; Domschke, K.; Neuner, M.; Jacob, C.P.; Buttenschon, H.N.; Boreatti-Hummer, A.; Volkert, J.; Herterich, S.; et al. Cross-disorder analysis of bipolar risk genes: Further evidence of DGKH as a risk gene for bipolar disorder, but also unipolar depression and adult ADHD. Neuropsychopharmacology 2011, 36, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, A.; Mamdani, F.; Ernst, C.; Vawter, M.P.; Bunney, W.E.; Lebel, V.; Rehal, S.; Klempan, T.; Gratton, A.; Benkelfat, C.; et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.L.; Hyde, T.M.; Deep-Soboslay, A.; Kleinman, J.E.; Sodhi, M.S. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol. Psychiatry 2015, 20, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; van der Veen, J.W.; Tumonis, T.; Meyers, N.; Shen, J.; Drevets, W.C. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 2007, 64, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xu, Y.; Zhou, L.; Zhang, C.; Meng, Q.; Wu, S.; Wang, S.; Ding, Z.; Chen, X.; Li, X.; et al. Sex-Dependent Depression-Like Behavior Induced by Respiratory Administration of Aluminum Oxide Nanoparticles. Int. J. Environ. Res. Public Health 2015, 12, 15692-15705. https://doi.org/10.3390/ijerph121215011
Zhang X, Xu Y, Zhou L, Zhang C, Meng Q, Wu S, Wang S, Ding Z, Chen X, Li X, et al. Sex-Dependent Depression-Like Behavior Induced by Respiratory Administration of Aluminum Oxide Nanoparticles. International Journal of Environmental Research and Public Health. 2015; 12(12):15692-15705. https://doi.org/10.3390/ijerph121215011
Chicago/Turabian StyleZhang, Xin, Yan Xu, Lian Zhou, Chengcheng Zhang, Qingtao Meng, Shenshen Wu, Shizhi Wang, Zhen Ding, Xiaodong Chen, Xiaobo Li, and et al. 2015. "Sex-Dependent Depression-Like Behavior Induced by Respiratory Administration of Aluminum Oxide Nanoparticles" International Journal of Environmental Research and Public Health 12, no. 12: 15692-15705. https://doi.org/10.3390/ijerph121215011





