As(V) and P Competitive Sorption on Soils, By-Products and Waste Materials
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
| Parameter | Forest Soil | Vineyard Soil | Pyritic Material | Granitic Material | Coarse Shell | Fine Shell | Shell Ash | Pine-Sawdust | Slate Fines |
|---|---|---|---|---|---|---|---|---|---|
| C (%) | 4.22 | 2.94 | 0.26 | 0.11 | 12.67 | 11.43 | 13.21 | 46.13 | 0.2 |
| N (%) | 0.33 | 0.23 | 0.04 | 0.04 | 0.36 | 0.21 | 1.13 | 0.03 | 0.02 |
| pHwater | 5.65 | 4.48 | 2.97 | 5.72 | 9.11 | 9.39 | 12.54 | 4.91 | 8.61 |
| Cae (cmol·kg−1) | 4.37 | 1.78 | 0.36 | 0.18 | 12.64 | 24.75 | 39.27 | 5.39 | 4.31 |
| Mge (cmol·kg−1) | 0.66 | 0.24 | 0.29 | 0.13 | 0.58 | 0.72 | 7.47 | 1.37 | 0.31 |
| Nae (cmol·kg−1) | 0.33 | 0.14 | 0.14 | 0.27 | 5.24 | 4.37 | 19.92 | 0.66 | 0.63 |
| Ke (cmol·kg−1) | 0.60 | 0.83 | 0.24 | 0.31 | 0.31 | 0.38 | 2.61 | 1.55 | 0.31 |
| Ale (cmol·kg−1) | 1.92 | 2.28 | 2.86 | 1.63 | 0.04 | 0.03 | 0 | 0.05 | 0.01 |
| CICe (cmol·kg−1) | 7.88 | 5.27 | 3.89 | 2.53 | 18.82 | 30.25 | 69.28 | 9.02 | 5.57 |
| POlsen (mg·kg−1) | 28.80 | 147.64 | 8.80 | 2.56 | 23.21 | 54.17 | 534.6 | 11.47 | 0.93 |
| PT (mg·kg−1) | 423.9 | 679.3 | 606.3 | 88.62 | 186.5 | 101.5 | 1617 | 88.04 | 661.3 |
| CaT (mg·kg−1) | 708.5 | 607.1 | 603 | <0.01 | 298,085 | 280,168 | 247,859 | 8088 | 2810 |
| MgT (mg·kg−1) | 830.5 | 5003 | 8384 | 355 | 1020 | 980.6 | 5286 | 164.4 | 11,797 |
| NaT (mg·kg−1) | 515.1 | 297.6 | 412 | 102 | 5508 | 5173 | 8074 | 98.35 | 53.72 |
| KT (mg·kg−1) | 1544 | 5441 | 3186 | 1434 | 80.57 | 202.1 | 896 | 540.7 | 991.3 |
| AsT (mg·kg−1) | 4.18 | 3.41 | 7 | 3 | 0.48 | 1.12 | 1.71 | 0.39 | 3.1 |
| CdT (mg·kg−1) | 0.43 | 0.14 | 0.08 | <0.001 | 0.02 | 0.07 | 63.09 | 50.82 | 95.18 |
| CrT (mg·kg−1) | 18.35 | 41.44 | 99 | 3 | 1.32 | 4.51 | 4596 | 234.2 | 54,010 |
| CuT (mg·kg−1) | 15.72 | 521.1 | 773 | 7 | 3.20 | 6.72 | 31.75 | 14.87 | 30.95 |
| NiT (mg·kg−1) | 10.69 | 21.73 | 5 | 1 | 5.64 | 8.16 | 3421 | 260.6 | 24,737 |
| ZnT (mg·kg−1) | 36.74 | 49.57 | 58 | 18 | 7.71 | 7.66 | 18.75 | 0 | 36.89 |
| MnT (mg·kg−1) | 92.99 | 305.4 | 296 | 24 | 5.70 | 33.75 | 18.67 | 5.19 | 28.46 |
| AlT (mg·kg−1) | 22,676 | 25,664 | 9624 | 5981 | 93.89 | 433.2 | 3421 | 260.7 | 24,737 |
| FeT (mg·kg−1) | 9486 | 21,284 | 135,157 | 3505 | 170.37 | 1855 | 4596 | 234.2 | 54,010 |
| Alo (mg·kg−1) | 4275 | 2003 | 563 | 1425 | 85.00 | 178.3 | 1733 | 112.5 | 730.6 |
| Feo (mg·kg−1) | 2333 | 1239 | 41,860 | 224 | 42.67 | 171.0 | 1659 | 15.62 | 1256 |
2.2. Methods
2.2.1. Characterization of the Materials Used
2.2.2. As(V) and P Competitive Sorption
3. Results
3.1. Sorbed As When a Constant P Concentration and Increasing As Concentrations Are Added, and Sorbed P When a Constant As Concentration and Increasing P Concentrations Are Added
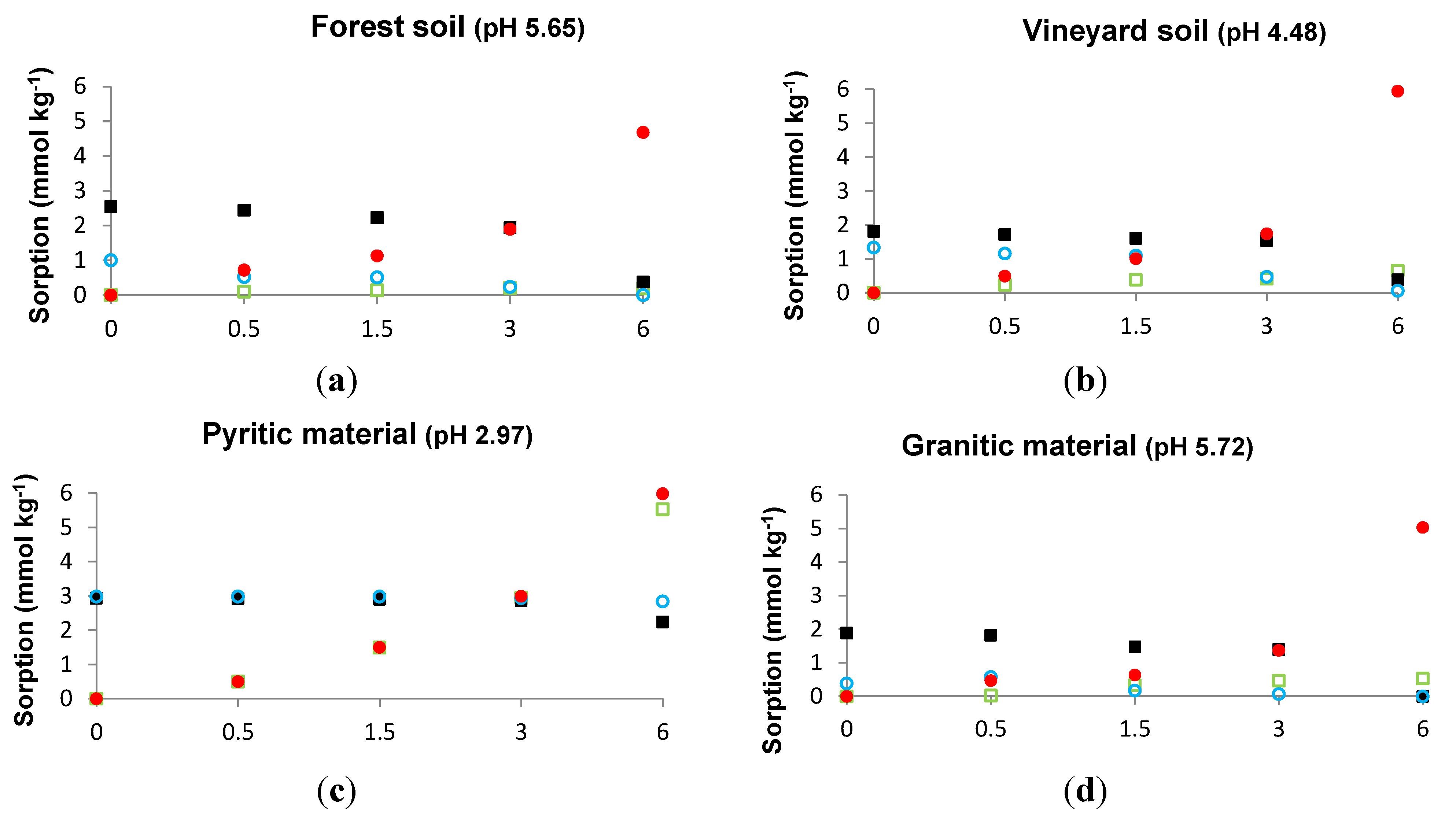
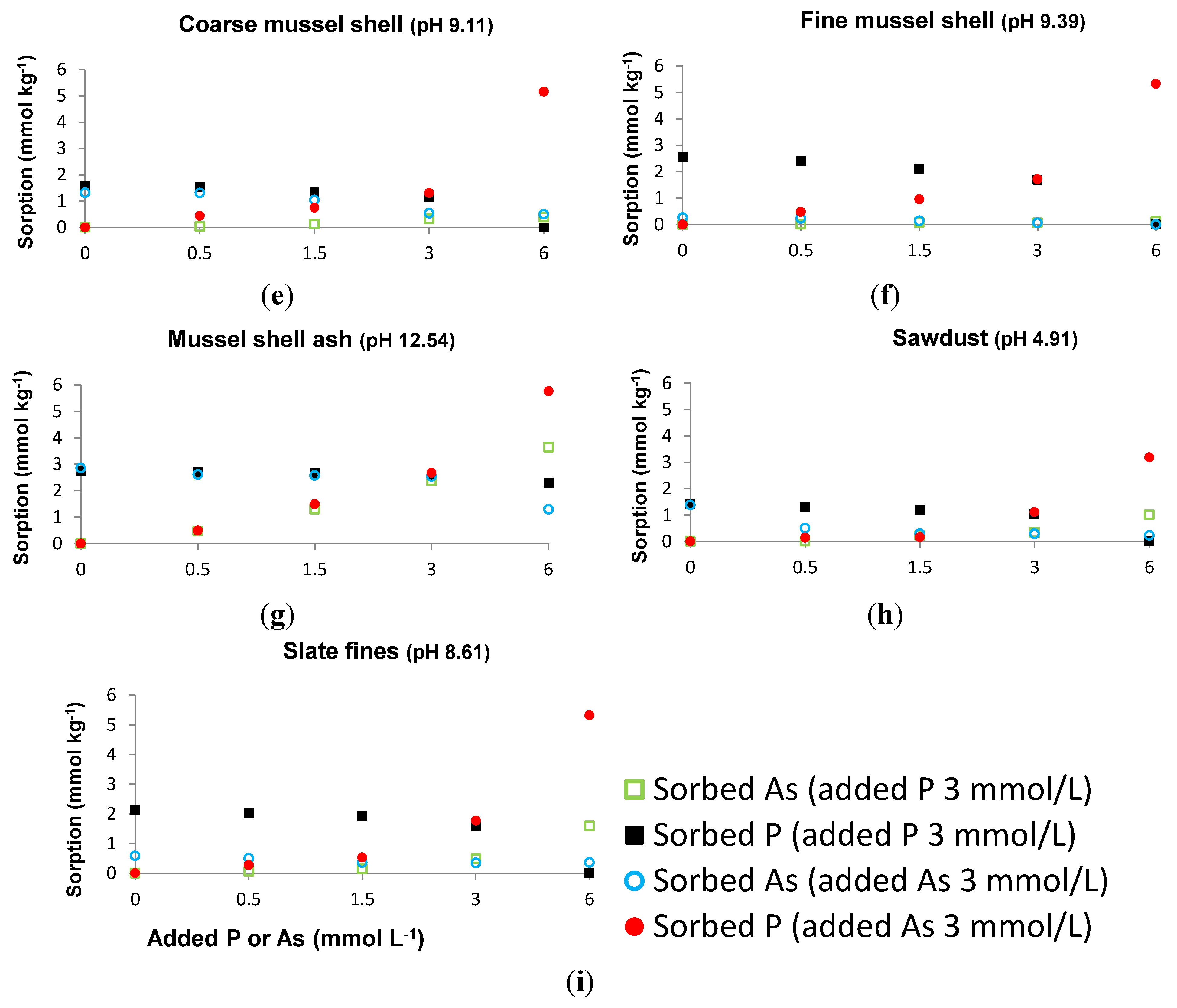
3.2. As and P Sorbed in the Absence or in the Presence of Each Other
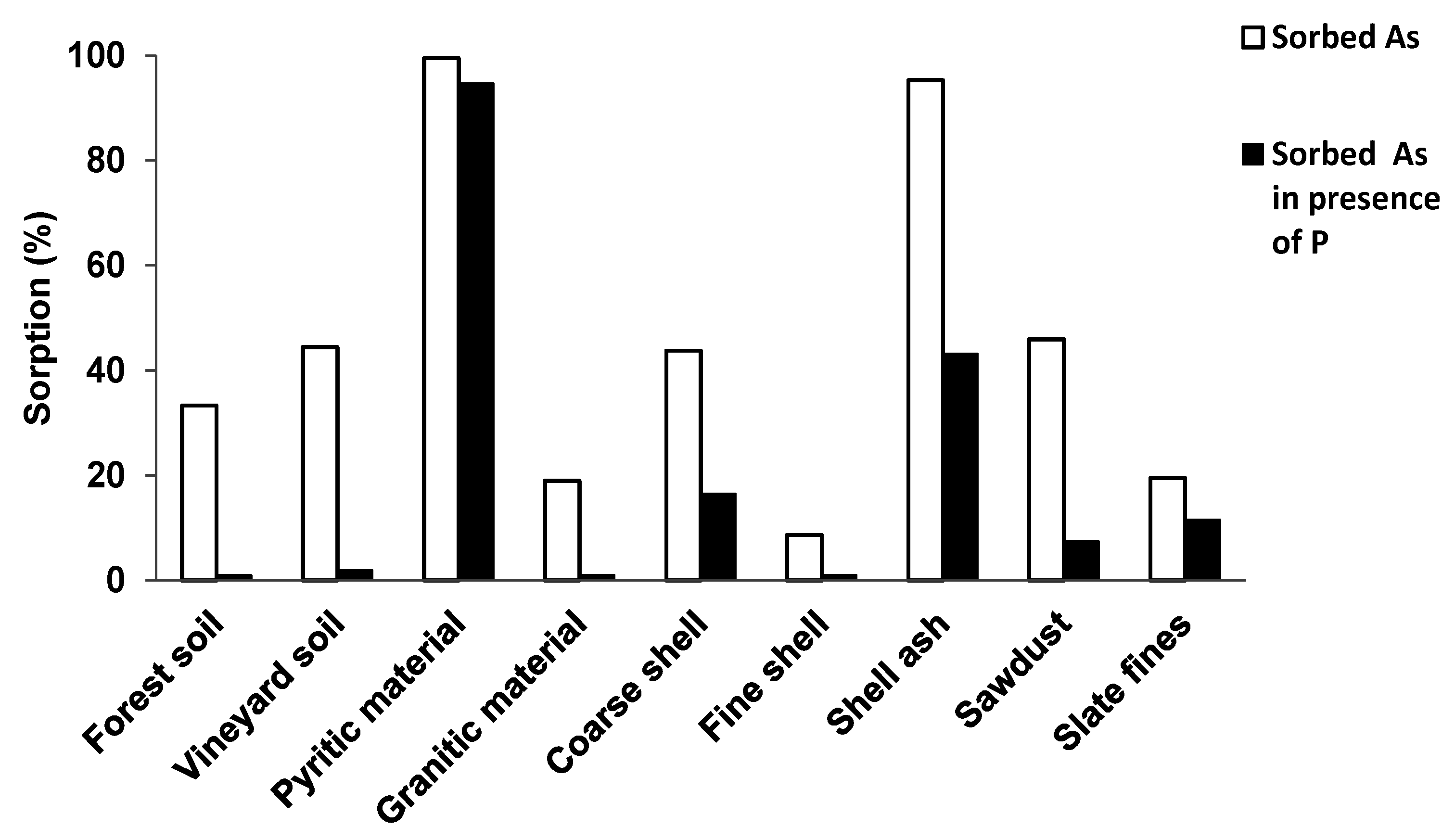

4. Discussion
4.1. As and P Competition
4.2. Implications of the Study
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, E.; Naidu, R.; Alston, A.M. Arsenic in the soil environment: A review. Adv. Agron. 1998, 64, 149–195. [Google Scholar]
- Gur, A.; Gil, Y.; Bravd, B. The efficacy of several herbicides in the vineyard and their toxicity to grapevines. Weed Res. 1979, 19, 109–116. [Google Scholar] [CrossRef]
- Clothier, B.E.; Green, S.R.; Vogeler, I.; Greven, M.M.; Agnew, R.; van den Dijssel, C.W.; Neal, S.; Robinson, B.H.; Davidson, P. CCA transport in soil from treated-timber posts, pattern dynamics from the local to regional scale. Hydrol. Earth Syst. Sci. Discus. 2006, 3, 2037–2061. [Google Scholar] [CrossRef]
- Thomas, M.L.; Lal, R.; Logan, T.; Fausey, N.R. Land use and management effects on nonpoint loading from Miamian soil. Soil Sci. Soc. Am. J. 1992, 56, 1871–1875. [Google Scholar] [CrossRef]
- Garnier, J.; Cugier, P.; Billen, G.; Guillaud, J.F.; Ménesguen, A. Modelling the eutrophication of the Seine Bight (France) under historical, present and future riverine nutrient loading. J. Hydrol. 2005, 304, 381–396. [Google Scholar]
- Zhang, H.; Selim, H.M. Competitive sorption-desorption kinetics of arsenate and phosphate in soil. Soil Sci. 2008, 173, 3–12. [Google Scholar] [CrossRef]
- Violante, A.; Pucci, M.; Cozzolino, V.; Zhu, J.; Pigna, M. Sorption/desorption of arsenate on/from Mg–Al layered double hydroxides: Influence of phosphate. J. Colloid. Interface Sci. 2009, 333, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wu, P.; Su, S.; Bai, L.; Feng, Q. Phosphate has a differential influence on arsenate adsorption by soils with different properties. Plant Soil Environ. 2012, 58, 405–411. [Google Scholar]
- Bowell, R.J. Sorption of arsenic by iron oxides and oxyhydroxides in soils. Appl. Geochem. 1994, 9, 279–286. [Google Scholar] [CrossRef]
- Zhang, H.; Selim, H.M. Kinetics of arsenate adsorption-desorption in soils. Environ. Sci. Technol. 2005, 39, 6101–6108. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, G.; Li, H. Efficient removal of arsenic from water using a granular adsorbent: Fe–Mn binary oxide impregnated chitosan bead. Bioresource Technol. 2015, 193, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Maguire, R.O.; Foy, R.H.; Baily, J.S.; Sims, J.T. Estimation of the phosphorus sorption capacity of acidic soils in Ireland. Eur. J. Soil Sci. 2001, 52, 479–487. [Google Scholar] [CrossRef]
- Rahnemaie, R.; Hiemstra, T.; van Riemsdijk, W.H. Geometry, charge distribution, and surface speciation of phosphate on goethite. Langmuir 2007, 23, 3680–3689. [Google Scholar] [CrossRef] [PubMed]
- Devau, N.; Le Cadre, E.; Hinsinger, P.; Gerard, F. A mechanistic model for understanding root-induced chemical changes controlling phosphorus availability. Ann. Bot. 2010, 105, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.E.; Neff, J.; Maybach, B.T.; Reynolds, R.L. Chemical and textural controls on phosphorus mobility in drylands of southeastern Utah. Biogeochemistry 2010, 100, 105–120. [Google Scholar] [CrossRef]
- Weng, P.; Vega, F.L.; Van Riemsdijk, W.H. Competitive and synergistic effects in pH dependent phosphate adsorption in soils: LCD modeling. Environ. Sci. Technol. 2011, 45, 8420–8428. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yu, X. Adsorption of fluoride, arsenate and phosphate in aqueous solution by cerium impregnated fibrous protein. Chem. Eng. J. 2012, 184, 205–212. [Google Scholar] [CrossRef]
- Castaldi, P.; Mele, E.; Silvetti, M.; Garau, G.; Deiana, S. Water treatment residues as accumulators of oxoanions in soil. Sorption, of arsenate and phosphate anions from an aqueous solution. J. Hazard. Mater. 2014, 264, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Neupane, G.; Donahoe, R.J.; Arai, Y. Kinetics of competitive adsorption/desorption of arsenate and phosphate at the ferrihydrite-water interface. Chem. Geol. 2014, 368, 31–38. [Google Scholar] [CrossRef]
- Parschova, H.; Asresahegnova, Z.; Jelinek, L.; Pohorela, A; Slapakova, P.; Sousa, H.; Mistova, E. The effect of accompanying anions on arsenate sorption onto selective sorbents. Separ. Sci. Technol. 2015, 50, 81–90. [Google Scholar] [CrossRef]
- Seco-Reigosa, N.; Bermúdez-Couso, A.; Garrido-Rodríguez, B.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V) retention on soils and forest by-products and other waste materials. Environ. Sci. Pollut. Res. 2013, 20, 6574–6583. [Google Scholar] [CrossRef] [PubMed]
- Osorio-López, C.; Seco-Reigosa, N.; Garrido-Rodríguez, B.; Cutillas-Barreiro, L.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V) adsorption on forest and vineyard soils and pyritic material with or without mussel shell: Kinetics and fractionation. J. Taiwan Inst. Chem. Eng. 2014, 45, 1007–1014. [Google Scholar] [CrossRef]
- Seco-Reigosa, N.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Mixtures including wastes from the mussel shell processing industry: retention of arsenic, chromium and mercury. J. Clean. Prod. 2014, 84, 680–690. [Google Scholar] [CrossRef]
- Otero, M.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Cr(VI) sorption/desorption on untreated and mussel-shell-treated soil materials: fractionation and effects of pH and chromium concentration. Solid Earth 2015, 6, 373–382. [Google Scholar] [CrossRef]
- Seco-Reigosa, N.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Adsorption, desorption and fractionation of As(V) on untreated and mussel shell-treated granitic material. Solid Earth 2015, 6, 337–346. [Google Scholar] [CrossRef]
- Chatterjee, A.; Lal, R.; Wielopolski, L.; Martin, M.Z.; Ebinger, M.H. Evaluation of different soil carbon determination methods. Cr. Rev. Plant Sci. 2009, 28, 164–178. [Google Scholar] [CrossRef]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; ASA: Madison, USA, 1982; pp. 199–223. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis, Part 3, Chemical Methods; ASA: Madison, USA, 1996; pp. 437–474. [Google Scholar]
- Kamprath, E.J. Exchangeable aluminium as a criterion for liming leached mineral soils. Soil Sci. Soc. Am. P. 1970, 34, 252–54. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; ASA: Madison, USA, 1982; pp. 403–430. [Google Scholar]
- Tan, K.H. Soil Sampling, Preparation and Analysis; Marcel-Decker: New York, NY, USA, 1996. [Google Scholar]
- Nóbrega, J.A.; Pirola, C.; Fialho, L.L.; Rota, G.; de Campos, C.E.K.M.A.J.; Pollo, F. Microwave-assisted digestion of organic samples: How simple can it become? Talanta 2012, 98, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.; Fernández-Sanjurjo, M.J.; Núñez, A.; Seco, N.; Corti, G. Aluminium fractionation and speciation in bulk and rhizosphere of a grass soil amended with mussel shells or lime. Geoderma 2013, 173, 322–329. [Google Scholar] [CrossRef]
- Murali, V.; Aylmore, L.A.G. Competitive adsorption during solute transport in soils: 3. A review of experimental evidence of competitive adsorption and an evaluation of simple competition models. Soil Sci. 1983, 136, 279–290. [Google Scholar] [CrossRef]
- Sø, H.U.; Postma, D.; Jakobsen, R.; Larsen, F. Competitive adsorption of arsenate and phosphate onto calcite; experimental results and modeling with CCM and CD-MUSIC. Geochim. Cosmochim. Acta 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Langmuir, D.; Mahoney, J.; Rowson, J. Solubility products of amorphous ferris arsenate and crystalline scorodite (FeAsO4·2H2O) and their application to arsenic behavior in buried mine tailings. Geochim. Cosmochim. Acta 2006, 70, 2942–2956. [Google Scholar] [CrossRef]
- Zhao, H.; Standford, R. Competitive adsorption of phosphate and arsenate on goethite. Environ. Sci. Technol. 2001, 35, 4753–4757. [Google Scholar]
- Gao, Y.; Mucci, A. Individual and competitive adsorption of phosphate and arsenate on goethite in artificial seawater. Chem. Geol. 2003, 199, 91–109. [Google Scholar] [CrossRef]
- Dixit, S.; Hering, J.G. Comparision of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, M.; Hiemstra, T.; van Riemsdijk, W.H. Multi-competitive interaction of As(III) and As(V) oxyanions with Ca2+, Mg2+, PO43−, and CO32− ions on goethite. J. Colloid. Interface Sci. 2008, 320, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.A.; Goldberg, S. Modeling arsenate competitive adsorption on kaolinite, montmorillonite and illite. Clays Clay Miner. 1996, 44, 609–623. [Google Scholar] [CrossRef]
- Violante, A.; Pigna, M. Competitive sorption of arsenate and phosphate on different clay minerals and soils. Soil Sci. Soc. Am. J. 2002, 66, 1788–1796. [Google Scholar] [CrossRef]
- Smith, E.; Naidu, R.; Alston, A.M. Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorption. J. Environ. Qual. 2002, 31, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Meinrath, G. Coordination of uranyl(VI) carbonate species in aqueous solutions. J. Radioanal. Nuclear Chem. 1996, 211, 349–362. [Google Scholar] [CrossRef]
- Khare, N.; Martin, J.D.; Hesterberg, D. Phosphate bonding configuration on ferrihydrite based on molecular orbital calculations and XANES fingerprint. Geochim. Cosmochim. Acta 2007, 71, 4405–4415. [Google Scholar] [CrossRef]
- Alexandratos, V.G.; Elzinga, E.J.; Reeder, R.J. Arsenate uptake by calcite: macroscopic and spectroscopic characterization of adsorption and incorporation mechanisms. Geochim. Cosmochim. Acta 2007, 71, 4172–4187. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Carreira, J.A.; Viiñegla, B.; Lajtha, K. Secondary CaCO3 and precipitation of P–Ca compounds control the retention of soil P in arid ecosystems. J. Arid Environ. 2006, 64, 460–473. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: New Jersey, NJ, USA, 2002. [Google Scholar]
- Wang, L.; Ruiz-Agudo, E.; Putnis, C.V.; Menneken, M.; Putnis, A. Kinetics of calcium phosphate nucleation and growth on calcite: Implications for predicting the fate of dissolved phosphate species in alkaline soils. Environ. Sci. Technol. 2012, 46, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Ranjbar, F. Aging effects on phosphorus transformation rate and fractionation in some calcareous soils. Geoderma 2009, 155, 101–106. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H. The adsorption of arsenic on magnetic iron-manganese oxide in aqueous medium. In Proceedings of the International Multi-Conference of Engineers and Computer Scientists, Vol II, Hong Kong, 9–21 March 2008; pp. 19–21.
- Mahuli, S.; Agnihotri, R.; Chauk, S.; Ghosh-Dastidar, A.; Fan, L.S. Mechanism of arsenic sorption by hydrated lime. Environ. Sci. Technol. 1997, 31, 3226–3231. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-Pérez, I.M.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V) and P Competitive Sorption on Soils, By-Products and Waste Materials. Int. J. Environ. Res. Public Health 2015, 12, 15706-15715. https://doi.org/10.3390/ijerph121215016
Rivas-Pérez IM, Paradelo-Núñez R, Nóvoa-Muñoz JC, Arias-Estévez M, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E, Núñez-Delgado A. As(V) and P Competitive Sorption on Soils, By-Products and Waste Materials. International Journal of Environmental Research and Public Health. 2015; 12(12):15706-15715. https://doi.org/10.3390/ijerph121215016
Chicago/Turabian StyleRivas-Pérez, Ivana María, Remigio Paradelo-Núñez, Juan Carlos Nóvoa-Muñoz, Manuel Arias-Estévez, María José Fernández-Sanjurjo, Esperanza Álvarez-Rodríguez, and Avelino Núñez-Delgado. 2015. "As(V) and P Competitive Sorption on Soils, By-Products and Waste Materials" International Journal of Environmental Research and Public Health 12, no. 12: 15706-15715. https://doi.org/10.3390/ijerph121215016








