The Geographic Distribution of Liver Cancer in Canada Does Not Associate with Cyanobacterial Toxin Exposure
Abstract
:1. Introduction
2. Methodology
2.1. Data Sources
| Variable | Source | Method of Calculation |
|---|---|---|
| Land Area devoted to Agriculture (%) | 2006 Canadian Census of Agriculture (accessed through the Data Liberation Initiative, University of Manitoba) | The reported area of land devoted to agriculture was divided by the total land area of each CD |
| Cattle Densities (no. of cattle/1000 sq. km) | 2006 Canadian Census of Agriculture (accessed through the Data Liberation Initiative, University of Manitoba) | The reported number of cattle in each CD was divided by the land area of each CD. |
| Swine Densities (no. of swine/100 sq. km) | 2006 Canadian Census of Agriculture (accessed through the Data Liberation Initiative, University of Manitoba) | The reported number of swine in each CD was divided by the land area of each CD |
| Population 40+ (%), 1996–2004 | Population Data supplied by National Cancer Registry | No. of persons aged 40 plus divided by the total population, using population denominator data from 1996–2004 combined |
| Male Population (%), 2001 | 2001 Canadian Census (accessed through the Data Liberation Initiative, University of Manitoba) http://www12.statcan.ca/english/census01/home/index.cfm | No. of males divided by the total Population |
| HepB Incidence, cases/100,000, 1989–2004 | Notifiable Diseases On-Line http://dsol-smed.phac-spc.gc.ca/dsolsmed/ndis/index_e.html | Reported HepB incidence for the years 1989-2004 were averaged at the provincial level. The average provincial rate was then applied to CDs by province. |
| HepC Incidence, cases/100 000, 1989–2004 | Notifiable Diseases On-Line http://dsol-smed.phac-spc.gc.ca/dsolsmed/ndis/index_e.html | Reported HepC incidence for the years 1989–2004 were averaged at the provincial level. The average provincial rate was then applied to CDs by province. |
| Alcohol Abuse (%), 40–64 yr olds, 2007 | 2007 Canadian Community Health Survey http://secure.cihi.ca/indicators/2007/en/datatables_maps07_e.html | Numerator (no. of excessive drinkers 40–64 years of age) and denominator (no. of persons 40–64 years of age) by Regional Health Authority Area were re-scaled to Census Division using the areal interpolation extension in Arc-GIS |
| Recent Immigrantion (%), 2001 | 2001 Canadian Census (accessed through the Data Liberation Initiative, University of Manitoba) http://www12.statcan.ca/english/census01/home/index.cfm | Derived directly from reported Census values |
| Urban/rural | 2001 DMTI Population Counts by City (accessed through the Data Liberation Initiative, University of Manitoba) http://www.dmtispatial.com/ | CD were classified as Urban if they contained at least one City with a population of 50,000 or more in 2001 |
2.2. Statistical Methods
3. Results
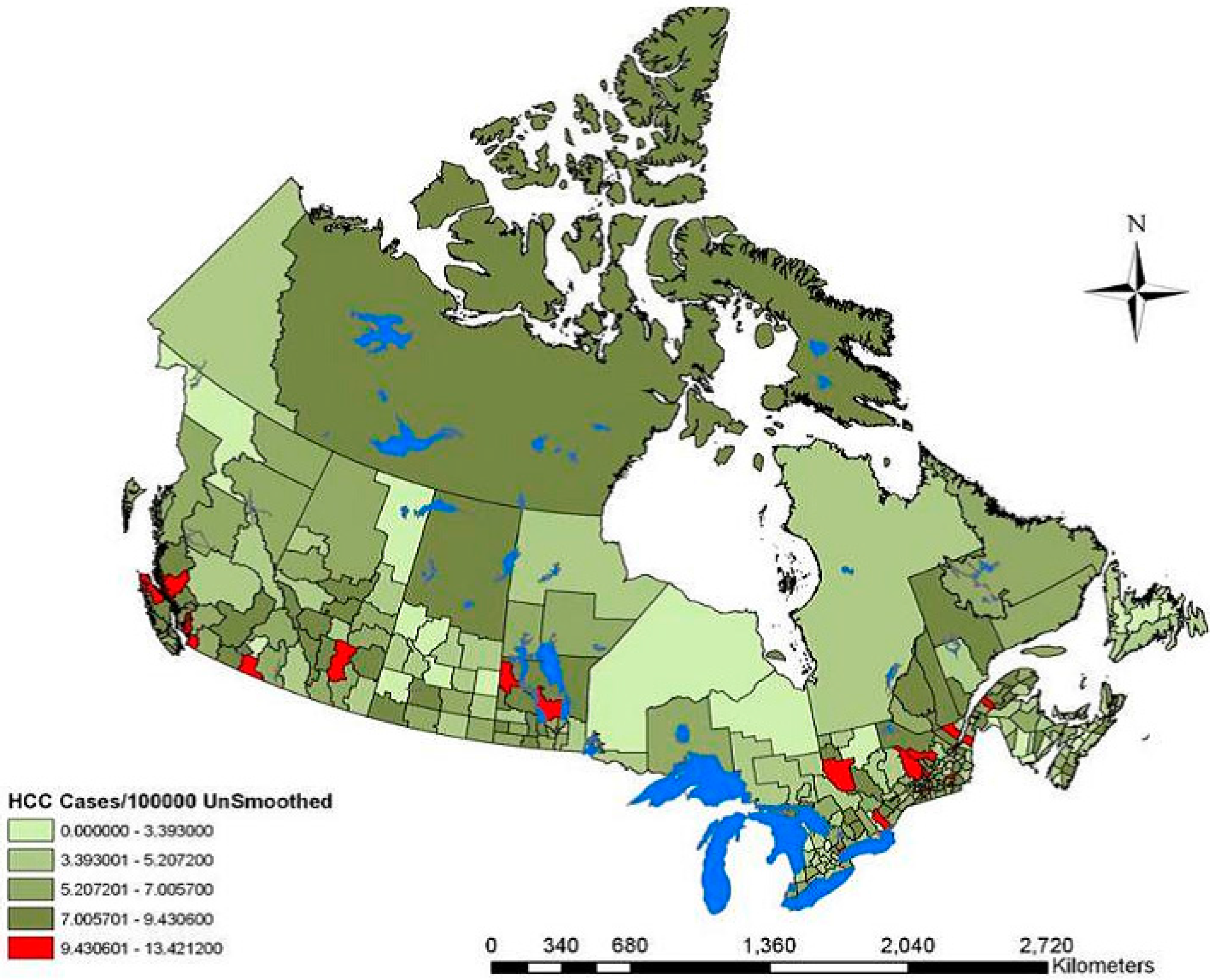

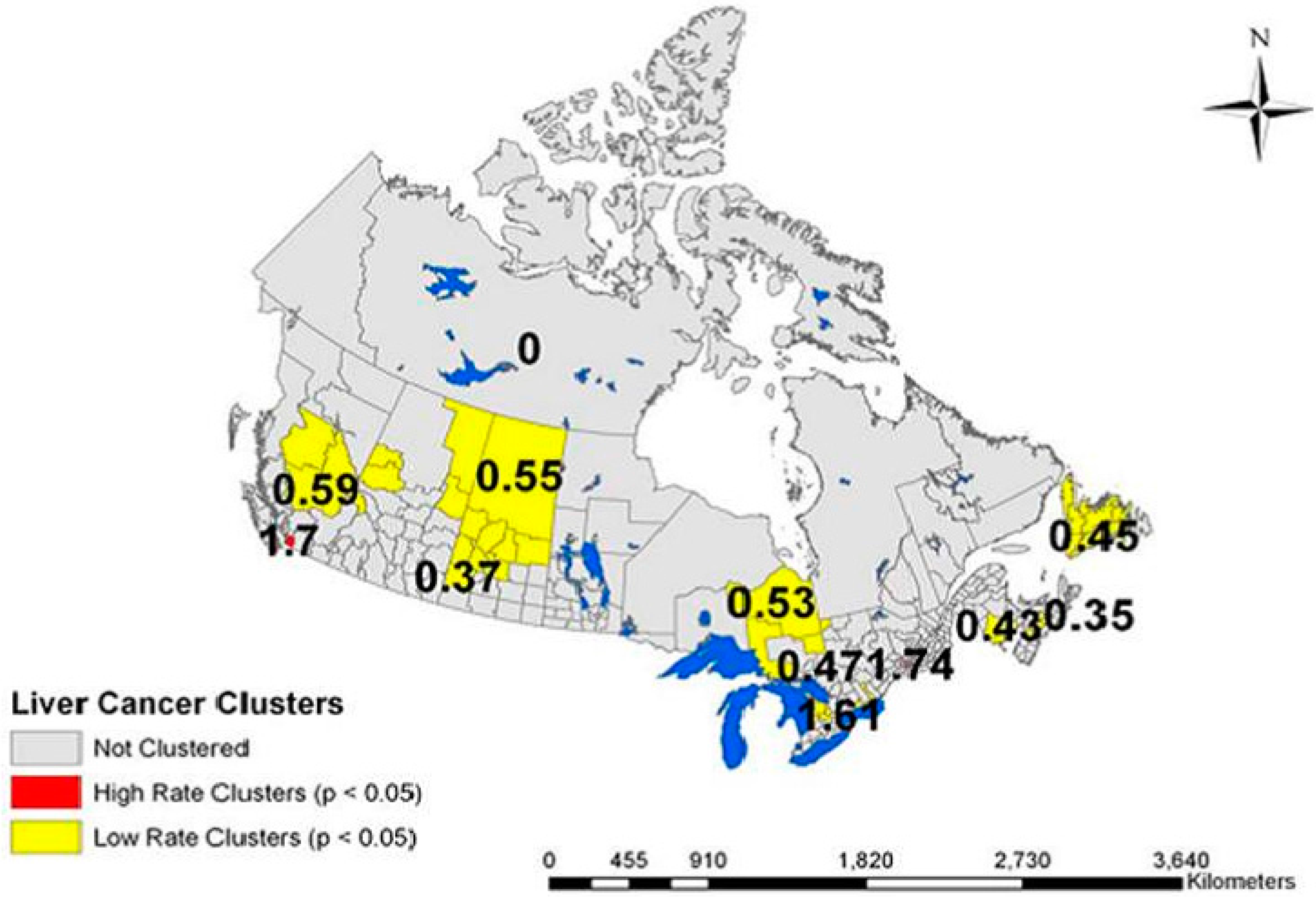
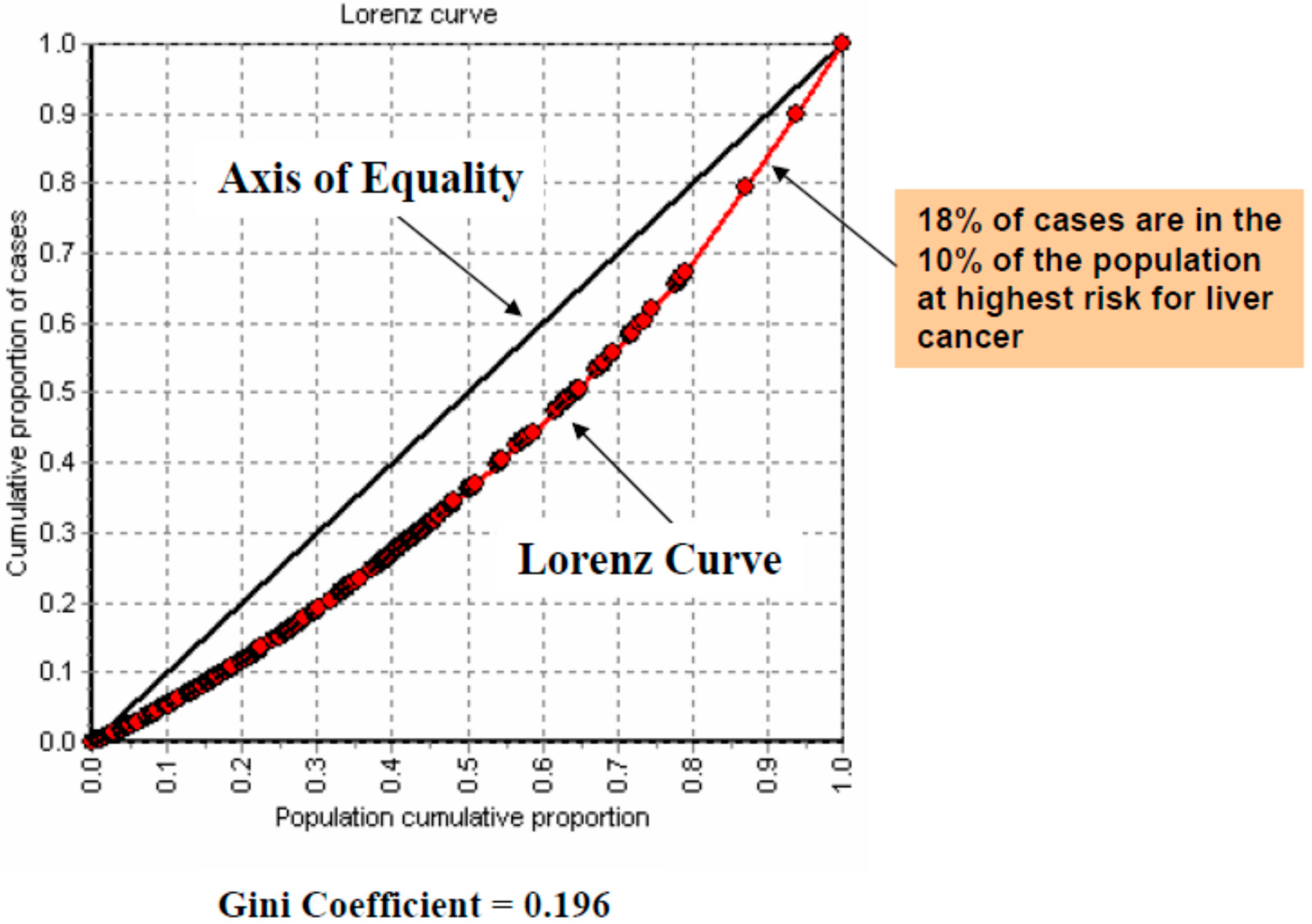
| Variable | Range | Overall RR (95% CI-RR) |
|---|---|---|
| Agriculture | >62.68 | 1.00 |
| (%) | 23.66–62.68 | 1.05 (0.92–1.20) |
| 2006 | <23.66 | 1.11 (0.98–1.25) |
| Cattle Density | <62,460 | 1.00 |
| Animals/1000 km2 | 62,460–262,169 | 0.96 (0.74–1.01) |
| 2006 | >262,169 | 1.27 (0.95–1.70) ** |
| Swine Density | <33.67 | 1.00 |
| Animals/1000 km2 | 33.67–142,937 | 0.93 (0.82–1.05) |
| 2006 | >142,937 | 1.00 (0.82–1.23) |
| Variable | Range | Overall RR (95% CI-RR) |
|---|---|---|
| Hepatitis B (Cases/100 000), 1989–2004 | <3.55 | 1.00 |
| 3.55–5.24 | 0.72 (0.61–0.65) ** | |
| >5.24 | 1.16 (1.06–1.27) ** | |
| Hepatitis C (Cases/100 000), 1989–2004 | <36.21 | 1.00 |
| 36.21–56.49 | 0.94 (0.85–1.05) | |
| >56.49 | 0.99 (0.87–1.27) | |
| Alcohol Abuse (%), 40–64 yrs, 2007 | <13.5 | 1.00 |
| 13.5–18.76 | 1.00 (0.89–1.13) | |
| >18.76 | 0.84 (0.72–0.99) * | |
| Recent Immigration (%), 2001 | <1.28 | 1.00 |
| 1.28–4.42 | 1.18 (1.05–1.33) ** | |
| >4.42 | 1.89 (1.58–2.27) ** | |
| Urban/Rural | Rural | 1.00 |
| Urban | 1.25 (1.12–1.40) ** | |
| Province | 10 Newfoundland | 1.00 |
| 24 Quebec | n/s | |
| 45 Manitoba | 1.73 (1.29–2.50) ** | |
| 48 Alberta | 1.60 (1.31–2.49) ** | |
| 59 British Columbia | 1.83 (1.33–2.57) ** | |
| 63 Nunavut | 2.22 (1.02–4.62) * |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pocobelli, G.; Cook, L.S.; Brant, R.; Lee, S.S. Hepatocellular carcinoma incidence trends in Canada: Analysis by birth cohort and period of diagnosis. Liver Int. 2008, 28, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Society Steering Committee. Canadian Cancer Statistics: 2010; Canadian Cancer Society: Toronto, ON, Canada, 2010. [Google Scholar]
- Marrett, L.D.; De, P.; Airia, P.; Dryer, D. Cancer in Canada in 2008. CMAJ 2008, 179, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Cancer Surveillance On-Line 2009. Available online: http://dsol-smed.phac-aspc.gc.ca/dsol-smed/cancer/c_prv-eng.php (accessed on 17 April 2011).
- Dyer, Z.; Peltekian, K.; van Zanten, S.V. Review article: The changing epidemiology of hepatocellular carcinoma in Canada. Aliment. Pharmacol. Ther. 2005, 22, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Corley, D.A. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am. J. Med. 2008, 121, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.; Barcak, E.; El-Serag, H.; Goodgame, R. The impact of immigration on the increasing incidence of hepatocellular carcinoma in the United States. Aliment. Pharmacol. Ther. 2004, 20, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.R.; Thein, H.H.; Gidding, H.F.; Amin, J.; Law, M.G.; George, J.; Dore, G.J. Risk factors for hepatocellular carcinoma in a cohort infected with hepatitis B or hepatitis C. J. Gastroenterol. Hepatol. 2011, 26, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Cabibbo, G.; Craxi, A. Epidemiology, risk factors and surveillance of hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 352–355. [Google Scholar] [PubMed]
- Loomba, R.; Yang, H.I.; Su, J.; Brenner, D.; Iloeje, U.; Chen, C.J. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin. Gastroenterol. Hepatol. 2010, 8, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Balbi, M.; Donadon, V.; Ghersetti, M.; Grazioli, S.; Valentina, G.D.; Gardenal, R.; Dal Mas, M.; Casarin, P.; Zanette, G.; Miranda, C. Alcohol and HCV chronic infection are risk cofactors of type 2 diabetes mellitus for hepatocellular carcinoma in Italy. Int. J. Environ. Res. Public Health 2010, 7, 1366–1378. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, A.; Boschi, L.; Del Gaudio, G.A.; Mastrangelo, L.; Munari, D. Liver damage in obese patients. Obes. Surg. 2002, 12, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W. Cyanobacteria secondary metabolites—The cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Puschner, B.; Galey, F.D.; Johnson, B.; Dickie, C.W.; Vondy, M.; Francis, T.; Holstege, D.M. Blue-green algae toxicosis in cattle. J. Am. Vet. Med. Assoc. 1998, 213, 1605–1607. [Google Scholar] [PubMed]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; E & FN Spon: London, UK, 1999. [Google Scholar]
- Jacoby, J.M.; Collier, D.C.; Welch, E.B.; Crayton, M. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can. J. Fish. Aquat. Sci. 2000, 57, 231–240. [Google Scholar] [CrossRef]
- Kotak, B.G.; Zurawell, R.W.; Prepas, E.E.; Holmes, C.F.B. Microcystin-LR concentration in aquatic food web compartments from lakes of varying trophic status. Can. J. Fish. Aquat. Sci. 1996, 53, 1974–1985. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S., 3rd; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [PubMed]
- Hudnell, K.H.; Paerl, H. Nutrient and other environmental controls of harmful cyanobacterial blooms along freshwater-marine continum. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer Science: New York, NY, USA, 2008; Volume 619. [Google Scholar]
- Health Canada. Blue-Green Algae (Cyanobacteria) and their Toxins 2008. Available online: http://www.hc-sc.gc.ca/ewh-semt/pubs/water-eau/cyanobacter-eng.php (accessed on 17 April 2011).
- Hudnell, K.H. Cyanobacterial harmful algal blooms: State of the science and research needs. In Advances in Experimental Medicine and Biology; Back, N.C., Ligtha, I.R., Lambris, A., Paoletti, J.D., Eds.; Springer: Berlin, Germany, 2008; pp. 217–238, 239–258, 259–274. [Google Scholar]
- Carmichael, W.W. Health effects of toxin-producing cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Poste, A.E.; Hecky, R.E.; Guildford, S.J. Evaluating microcystin exposure risk through fish consumption. Environ. Sci. Technol. 2011, 45, 5806–5811. [Google Scholar] [CrossRef] [PubMed]
- Duy, T.N.; Lam, P.K.; Shaw, G.R.; Connell, D.W. Toxicology and risk assessment of freshwater cyanobacterial (blue-green algal) toxins in water. Rev. Environ. Contam. Toxicol. 2000, 163, 113–185. [Google Scholar] [PubMed]
- Gan, N.; Sun, X.; Song, L. Activation of Nrf2 by microcystin-LR provides advantages for liver cancer cell growth. Chem. Res. Toxicol. 2010, 23, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.; Matos, P.; Pereira, P.; Batoréu, M.C.; Silva, M.J.; Jordan, P. Microcystin-LR activates the ERK1/2 kinases and stimulates the proliferation of the monkey kidney-derived cell line Vero-E6. Toxicol. In Vitro 2010, 24, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Zegura, B.; Lah, T.T.; Filipic, M. The role of reactive oxygen species in microcystin-LR-induced DNA damage. Toxicology 2004, 200, 59–68. [Google Scholar] [PubMed]
- Ohta, T.; Nishiwaki, R.; Yatsunami, J.; Komori, A.; Suganuma, M.; Fujiki, H. Hyperphosphorylation of cytokeratins 8 and 18 by microcystin-LR, a new liver tumor promoter, in primary cultured rat hepatocytes. Carcinogenesis 1992, 13, 2443–2447. [Google Scholar] [CrossRef] [PubMed]
- Monks, N.R.; Liu, S.; Xu, Y.; Yu, H.; Bendelow, A.S.; Moscow, J.A. Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1- and OATP1B3-expressing HeLa cells. Mol. Cancer Ther. 2007, 6, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Solter, P.F.; Wollenberg, G.K.; Huang, X.; Chu, F.S.; Runnegar, M.T. Prolonged sublethal exposure to the protein phosphatase inhibitor microcystin-LR results in multiple dose-dependent hepatotoxic effects. Toxicol. Sci. 1998, 44, 87–96. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Ueno, Y.; Nagata, S.; Tsutsumi, T.; Hasegawa, A.; Watanabe, M.F.; Park, H.D.; Chen, G.C.; Chen, G.; Yu, S.Z. Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis 1996, 17, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Z.; Chen, G. Blue-green algae toxins and liver cancer. Chin. J. Cancer Res. 1994, 6, 9–17. [Google Scholar]
- Svircev, Z.; Krstic, S.; Miladinov-Mikov, M.; Baltic, V.; Vidovic, M. Freshwater cyanobacterial blooms and primary liver cancer epidemiological studies in Serbia. J. Environ. Sci. Health 2009, 27, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Fleming, L.E.; Rivero, C.; Burns, J.; Williams, C.; Bean, J.A.; Shea, K.A.; Stinn, J. Blue green algal (cyanobacterial) toxins, surface drinking water, and liver cancer in Florida. Harmful Algae 2002, 1, 157–168. [Google Scholar] [CrossRef]
- Lake Champlain Basin Program. Blue green Algae Monitoring Program. 2000. Available online: http://www.lcbp.org/bgalgae.htm (accessed on 25 October 2011).
- Bunting, L.; Leavitt, P.R.; Wissel, B.; Laird, K.R.; Cumming, B.F.; St. Amand, A.; Engstrom, D.R. Sudden Ecosystem State Change in Lake Winnipeg, Canada, Caused by Eutrophicationarising from Crop and Livestock Production during the 20th Century; Government of Manitoba: Winnipeg, MB, Canada, 2011; pp. 1–67.
- Casey, A. Forgotten Lake. Available online: http://www.canadiangeographic.ca/magazine/nd06/feature_lake_winnipeg.asp (accessed on 26 November 2015).
- Large Lakes and Rivers Forecasting Research Branch. Detroit River-Western Lake Erie Basin Indicator Project. 2009. Available online: http://www.epa.gov/med/grosseile_site/indicators/algae-blooms.html (accessed on 1 November 2011). [Google Scholar]
- Nordenstedt, H.; White, D.L.; El-Serag, H.B. The changing pattern of epidemiology in hepatocellular carcinoma. Dig. Liver Dis. 2010, 42 (Suppl. S3), S206–S214. [Google Scholar] [CrossRef]
- ESRI. ArcGis 9.3 Geocoding Technology; ESRI 380 New York St.: Redland, CA, USA, 2009. [Google Scholar]
- GeoDa. Center for Geospatial Analysis and Computation, A.S.U.; GeoDa: Tempe, AZ, USA, 2006. [Google Scholar]
- Kulldorff, M. Satscan 8.0, Software for Spatial and Space-Time Scan Statistics; Information Management Services Inc.: Boston, MA, USA, 2009. [Google Scholar]
- Comesana, A.B.; Cerqueiro, J.F.; Medina, R.S. EPIDAT 3.0: Epidemiological Analysis from Tabulated Data; Pan American Health Organization, Conselleria de Sanidade: A Coruña, Spain, 2005. [Google Scholar]
- SAS Institute Inc. Sas 9.13 ETL Studio; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Yu, S.Z. Primary prevention of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1995, 10, 674–682. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labine, M.A.; Green, C.; Mak, G.; Xue, L.; Nowatzki, J.; Griffith, J.; Minuk, G.Y. The Geographic Distribution of Liver Cancer in Canada Does Not Associate with Cyanobacterial Toxin Exposure. Int. J. Environ. Res. Public Health 2015, 12, 15143-15153. https://doi.org/10.3390/ijerph121214969
Labine MA, Green C, Mak G, Xue L, Nowatzki J, Griffith J, Minuk GY. The Geographic Distribution of Liver Cancer in Canada Does Not Associate with Cyanobacterial Toxin Exposure. International Journal of Environmental Research and Public Health. 2015; 12(12):15143-15153. https://doi.org/10.3390/ijerph121214969
Chicago/Turabian StyleLabine, Meaghan A., Chris Green, Giselle Mak, Lin Xue, Janet Nowatzki, Jane Griffith, and Gerald Y. Minuk. 2015. "The Geographic Distribution of Liver Cancer in Canada Does Not Associate with Cyanobacterial Toxin Exposure" International Journal of Environmental Research and Public Health 12, no. 12: 15143-15153. https://doi.org/10.3390/ijerph121214969





