Associations between Carotid Artery Plaque Score, Carotid Hemodynamics and Coronary Heart Disease
Abstract
:1. Introduction
2. Methods
2.1. Patient Population and Selection
2.2. Carotid Ultrasonography
2.3. Coronary Angiography
3. Statistical Analysis
4. Results
4.1. Demographic Characteristics and Clinical Parameters between CHD and Non-CHD Groups
| Characteristics | CHD (n = 321) | Non-CHD (n = 155) | x2/t | P-Value | ||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age(years) | 45–59 years | 57 (18.51%) | 37 (25.69%) | 4.88 | 0.027 | |
| 60–74 years | 172 (55.84%) | 81 (56.25%) | ||||
| 75–89 years | 79 (25.65%) | 26 (18.06%) | ||||
| Sex | male | 180 (57.14%) | 62 (41.89%) | 9.38 | 0.002 | |
| female | 135 (42.86%) | 86 (58.11%) | ||||
| BMI | 19.64 ± 3.00 | 20.11 ± 2.87 | 1.12 | 0.266 | ||
| Register | rural | 194 (61.59%) | 87 (58.78%) | 0.33 | 0.490 | |
| urban | 121 (38.41%) | 61 (41.22%) | ||||
| Education | under middle school | 214 (67.94%) | 98 (66.22%) | 0.14 | 0.712 | |
| middle school | 62 (19.68%) | 31 (20.95%) | ||||
| beyond middle school | 39 (12.38%) | 19 (12.84%) | ||||
| Smoking | Yes | 46 (31.08%) | 14 (19.44%) | 2.02 | 0.069 | |
| Hypertension | Yes | 107 (72.30%) | 48 (66.67%) | 0.74 | 0.390 | |
| Hyperlipidemia | Yes | 15 (10.14%) | 4 (5.56%) | 2.88 | 0.237 | |
| DM | Yes | 33 (22.30%) | 24 (33.33%) | 3.07 | 0.080 | |
| TC | 4.05 ± 0.92 | 4.19 ± 0.92 | 1.03 | 0.304 | ||
| TG | 1.46 ± 0.85 | 1.22 ± 0.56 | 1.12 | 0.264 | ||
| LDL | 2.41 ± 3.00 | 2.42 ± 0.66 | 0.05 | 0.970 | ||
| Apo a | 1.44 ± 0.21 | 1.22 ± 0.21 | −0.51 | 0.614 | ||
| Apo b | 1.01 ± 0.24 | 0.84 ± 0.22 | −0.5 | 0.612 | ||
| Hcy | 14.78 ± 11.58 | 12.74 ± 5.72 | −1.25 | 0.199 | ||
| Ultrasound Parameters | ||||||
| IMT (mm) | CCA | 0.94 ± 0.14 | 0.89 ± 0.20 | −1.05 | 0.326 | |
| ICA | 0.83 ± 0.31 | 0.77 ± 0.16 | −2.14 | 0.033 | ||
| CAB | 1.65 ± 0.82 | 1.31 ± 0.60 | −3.83 | 0.000 | ||
| PS | 2.79 ± 2.37 | 1.57 ± 2.46 | −4.93 | 0.000 | ||
| Peak systolic velocity (PSV) | CCA | left | 65.28 ± 18.98 | 64.63 ± 18.20 | −0.38 | 0.701 |
| right | 63.14 ± 18.98 | 65.23 ± 18.20 | 1.40 | 0.250 | ||
| ICA | left | 52.17 ± 16.00 | 51.06 ± 14.92 | −0.49 | 0.644 | |
| right | 53.64 ± 18.98 | 49.93 ± 18.20 | −2.08 | 0.017 | ||
| End diastolic velocity (EDV) | CCA | left | 17.55 ± 6.08 | 18.43 ± 6.98 | 2.40 | 0.464 |
| right | 16.84 ± 18.98 | 17.77 ± 18.20 | 1.40 | 0.159 | ||
| ICA | left | 19.69 ± 6.44 | 19.12 ± 7.02 | −0.60 | 0.553 | |
| right | 19.28 ± 18.98 | 18.63 ± 18.20 | −1.07 | 0.227 | ||
| Resistance index (RI) | CCA | left | 0.73 ± 0.06 | 0.71 ± 0.06 | −2.57 | 0.010 |
| right | 0.73 ± 0.06 | 0.71 ± 0.06 | −2.16 | 0.032 | ||
| ICA | left | 0.62 ± 0.07 | 0.61 ± 0.06 | −1.12 | 0.263 | |
| right | 0.64 ± 0.07 | 0.61 ± 0.06 | 2.40 | 0.001 | ||
4.2. Multiple Logistic Analysis for CHD
| Factors | β | B | S.E. | χ2 | P-Value | OR (95% CI) |
|---|---|---|---|---|---|---|
| Age | 0.04 | 0.22 | 0.29 | 5.81 | 0.02 | 1.104 (1.008, 1.078) |
| PSV of right ICA | 0.02 | 0.21 | 0.01 | 4.28 | 0.04 | 1.021 (1.001, 1.041) |
| PS | 0.47 | 0.57 | 0.11 | 17.99 | <0.01 | 1.566 (1.273, 1.926) |
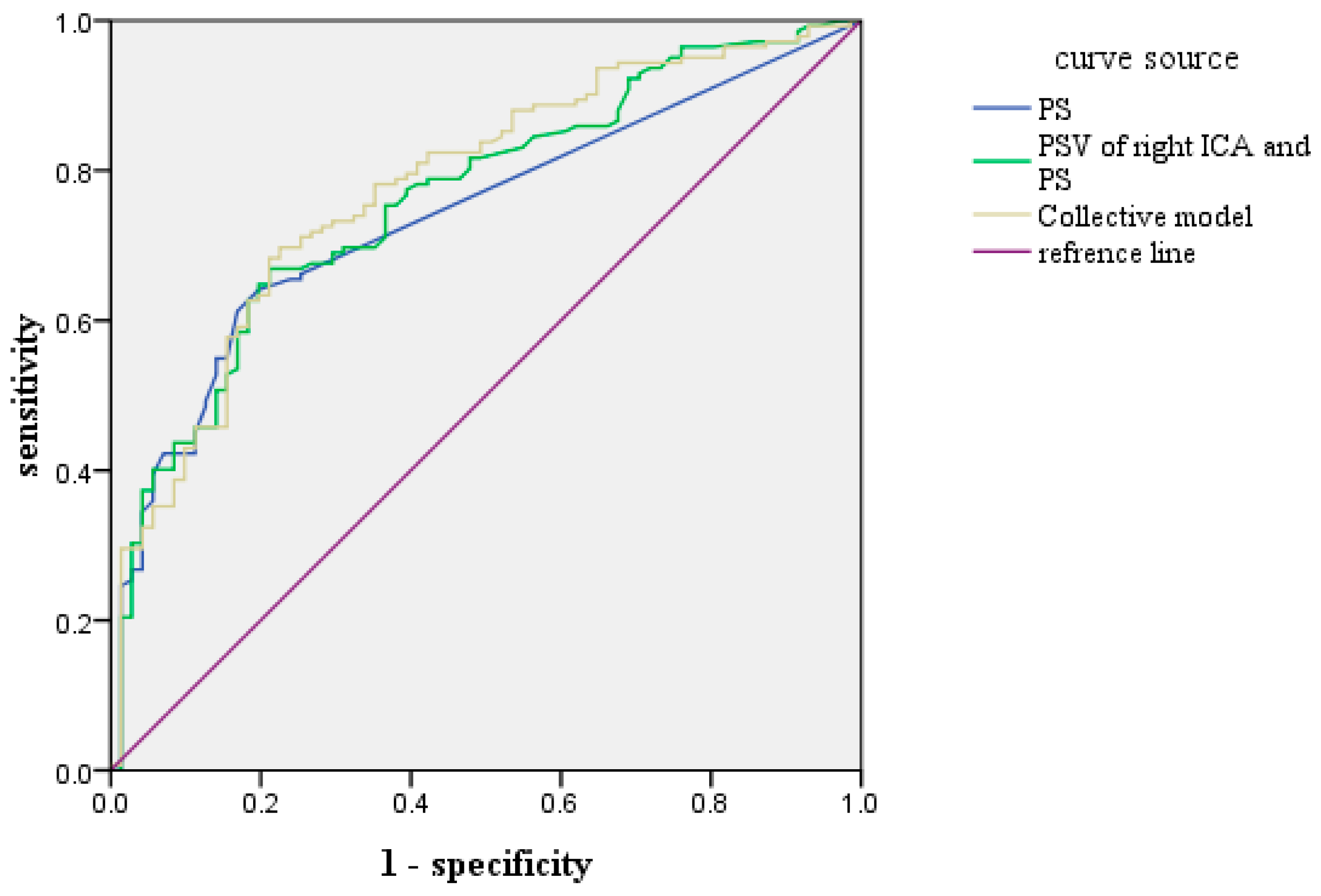
4.3. ROC Prediction Curve of Each Index of CHD
| Index | AUC | S.E | P-Value | 95% CI | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| PS | 0.740 | 0.034 | 0.000 | 0.673–0.807 | 0.613 | 0.831 |
| PS + PSV of right ICA | 0.762 | 0.033 | 0.0000 | 0.697–0.828 | 0.754 | 0.620 |
| PS + PSV of right ICA + Age | 0.778 | 0.030 | 0.000 | 0.719–0.847 | 0.782 | 0.648 |
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, G.; Wang, D.; Li, W.; Pan, Y.; Zheng, W.; Zhang, H.; Sun, Y.V. Coronary heart disease mortality in China: Age, gender, and urbanerural gaps during epidemiological transition. Rev. Panam. Salud Publica. 2012, 31, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, R.; Meng, X.; Zhao, Z.; Cai, J.; Wang, C.; Yang, C.; Kan, H. Short-term exposure to ambient air pollution and coronary heart disease mortality in 8 Chinese cities. Int. J. Cardiol. 2015, 197, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Zhao, D.; Gu, D.; Coxson, P.; Chen, C.S.; Cheng, J.; Liu, J.; He, J.; Goldman, L. The future impact of population growth and aging on coronary heart disease in China: Projections from the Coronary Heart Disease Policy Model-China. BMC Public Health. 2008. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; de Backer, G.; de Bacquer, D.; Ducimetière, P.; Jousilahti, P.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Brindle, P.; Emberson, J.; Lampe, F.; Walker, M.; Whincup, P.; Fahey, T.; Ebrahim, S. Predictive accuracy of the Framingham coronary risk score in British men: Prospective cohort study. BMJ. 2003. [Google Scholar] [CrossRef] [PubMed]
- Rundek, T.; Arif, H.; Boden-Albala, B.; Elkind, M.S.; Paik, M.C.; Sacco, R.L. Carotid plaque, a subclinical precursorof vascular events: The Northern Manhattan Study. Neurology. 2008, 70, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Chambless, L.E.; Heiss, G.; Folsom, A.R.; Rosamond, W.; Szklo, M.; Sharrett, A.R.; Clegg, L.X. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am. J. Epidemiol. 1997, 146, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Kolodgie, F.D.; Virmani, R. Correlation between carotid intimal/medial thickness and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D. Carotid ultrasound phenotypes are biologically distinct. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1910–1913. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of Clinical cardiovascular events with carotid intima-media thickness. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Polak, J.F.; Kavousi, M.; Mathiesen, E.B.; Völzke, H.; Tuomainen, T.P.; Sander, D.; Plichart, M.; Catapano, A.L.; Robertson, C.M.; et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project). Lancet 2012, 379, 2053–2062. [Google Scholar] [CrossRef]
- Costanzo, P.; Perrone-Filardi, P.; Vassallo, E.; Paolillo, S.; Cesarano, P.; Brevetti, G.; Chiariello, M. Does carotid intima-media thickness regression predict reduction of cardiovascular events? J. Am. Coll. Cardiol. 2010, 56, 2006–2020. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.H.; Mathiesen, E.B.; Joakimsen, O.; Stensland, E.; Wilsgaard, T.; Løchen, M.L.; Njølstad, I.; Arnesen, E. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: A 6-year follow-up study of 6226 persons: The Tromso Study. Stroke 2007, 38, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Tian, L. Predicting cardiovascular risk: So what do we do now? Arch. Intern. Med. 2006, 166, 1342–1344. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; de Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, W.M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Int. J. Behav. Med. 2012, 19, 403–488. [Google Scholar] [PubMed]
- Gonzalez-Juanatey, C.; Llorca, J.; Martin, J.; Gonzalez-Gay, M.A. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 2009, 38, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.J.; Sharrett, A.R.; Chambless, L.E.; Folsom, A.R.; Evans, G.W.; Heiss, G. Acoustic shadowing on B-mode ultrasound of the carotid artery predicts CHD. Ultrasound Med. Biol. 2001, 27, 357–365. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Greenland, P.; Arnett, D.K.; Ballantyne, C.M.; Criqui, M.H.; Elkind, M.S.; Go, A.S.; Harrell, F.E., Jr.; Hong, Y.; Howard, B.V.; et al. Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American Heart Association. Circulation 2009, 119, 2408–2016. [Google Scholar] [CrossRef] [PubMed]
- Longest, P.W.; Kleinstreuer, C.; Deanda, A. Numerical simulation of wall shear stress and particle-based hemodynamic parameters in pre-cuffed and streamlined end-to-side anastomoses. Ann. Biomed. Eng. 2005, 33, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Kleinstreuer, C.; Hyun, S.; Buchanan, J.R., Jr.; Longest, P.W.; Archie, J.P., Jr.; Truskey, G.A. Hemodynamic parameters and early intimal thickening in branching blood vessels. Crit. Rev. Biomed. Eng. 2001, 29, 1–64. [Google Scholar] [PubMed]
- Dickson, B.C.; Gotlieb, A.I. Towards understanding acute destabilization of vulnerable atherosclerotic plaques. Cardiovasc. Pathol. 2003, 12, 237–248. [Google Scholar] [CrossRef]
- Morito, N.; Inoue, Y.; Urata, M.; Yahiro, E.; Kodama, S.; Fukuda, N.; Saito, N.; Tsuchiya, Y.; Mihara, H.; Yamanouchi, Y.; et al. Increased carotid artery plaque score is an independent predictor of the presence and severity of coronary artery disease. J. Cardiol. 2008, 51, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.; Oshinski, J.N.; Giddens, D.P. Blood flow patterns in the proximal human coronary arteries: Relationship to atherosclerotic plaque occurrence. Mol. Cell. Biomech. 2008, 5, 9–18. [Google Scholar] [PubMed]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011). Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Yerly, P.; Rodondi, N.; Viswanathan, B.; Riesen, W.; Vogt, P.; Bovet, P. Association between conventional risk factors and different ultrasound-based markers of atherosclerosis at carotid and femoral levels in a middle-aged population. Int. J. Cardiovasc. Imaging. 2013, 29, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.J.; Harper, R.W.; Nestel, P.J. Severity of coronary atherosclerosis related to lipoprotein concentration. Br. Med. J. 1978, 2, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Gensini, G.G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 1983, 51, 606–608. [Google Scholar] [CrossRef]
- Yerly, P.; Marquès-Vidal, P.; Owlya, R.; Eeckhout, E.; Kappenberger, L.; Darioli, R.; Depairon, M. The Atherosclerosis Burden Score (ABS): A convenient ultrasound-based score of peripheral atherosclerosis for coronary artery disease prediction. J. Cardiovasc. Transl. Res. 2015, 8, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.B.; Park, H.W.; Jung, I.J.; Kim, W.H.; Kim, K.H.; Yang, D.J.; Park, Y.H.; Kim, Y.K.; Song, I.G.; Bae, J.H. Analysis of carotid ultrasound findings on cardiovascular events in patients with coronary artery disease during seven-year follow-up. Korean Circ. J. 2015, 45, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Satiroglu, O.; Kocaman, S.A.; Karadag, Z.; Temiz, A.; Cetin, M.; Canga, A.; Erdogan, T.; Bostan, M.; Cicek, Y.; Durakoglugil, E.; et al. Relationship of the angiographic extent of peripheral arterial disease with coronary artery involvement. J. Pak. Med. Assoc. 2012, 62, 644–649. [Google Scholar] [PubMed]
- Weber, T.; Wassertheurer, S.; O’Rourke, M.F.; Haiden, A.; Zweiker, R.; Rammer, M.; Hametner, B.; Eber, B. Pulsatile hemodynamics in patients with exertional dyspnea: Potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2013, 61, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Terzi, S.; Sayar, N.; Bilsel, T.; Enc, Y.; Yildirim, A.; Ciloğlu, F.; Yesilcimen, K. Tissue Doppler imaging adds incremental value in predicting exercise capacity in patients with congestive heart failure. Heart Vessels. 2007, 22, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Inabaa, Y.; Chen, J.A.; Bergmannb, S.R. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis 2012, 220, 128–133. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, M.; Ren, T.; Wang, X.; Liu, D.; Xu, M.; Han, L.; Wu, Z.; Li, H.; Zhu, Y.; et al. Associations between Carotid Artery Plaque Score, Carotid Hemodynamics and Coronary Heart Disease. Int. J. Environ. Res. Public Health 2015, 12, 14275-14284. https://doi.org/10.3390/ijerph121114275
Zhang H, Liu M, Ren T, Wang X, Liu D, Xu M, Han L, Wu Z, Li H, Zhu Y, et al. Associations between Carotid Artery Plaque Score, Carotid Hemodynamics and Coronary Heart Disease. International Journal of Environmental Research and Public Health. 2015; 12(11):14275-14284. https://doi.org/10.3390/ijerph121114275
Chicago/Turabian StyleZhang, Huiping, Mengxue Liu, Tiantian Ren, Xiangqian Wang, Dandan Liu, Mingliang Xu, LingFei Han, Zewei Wu, Haibo Li, Yu Zhu, and et al. 2015. "Associations between Carotid Artery Plaque Score, Carotid Hemodynamics and Coronary Heart Disease" International Journal of Environmental Research and Public Health 12, no. 11: 14275-14284. https://doi.org/10.3390/ijerph121114275





