Specific Association of Teratogen and Toxicant Metals in Hair of Newborns with Congenital Birth Defects or Developmentally Premature Birth in a Cohort of Couples with Documented Parental Exposure to Military Attacks: Observational Study at Al Shifa Hospital, Gaza, Palestine
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
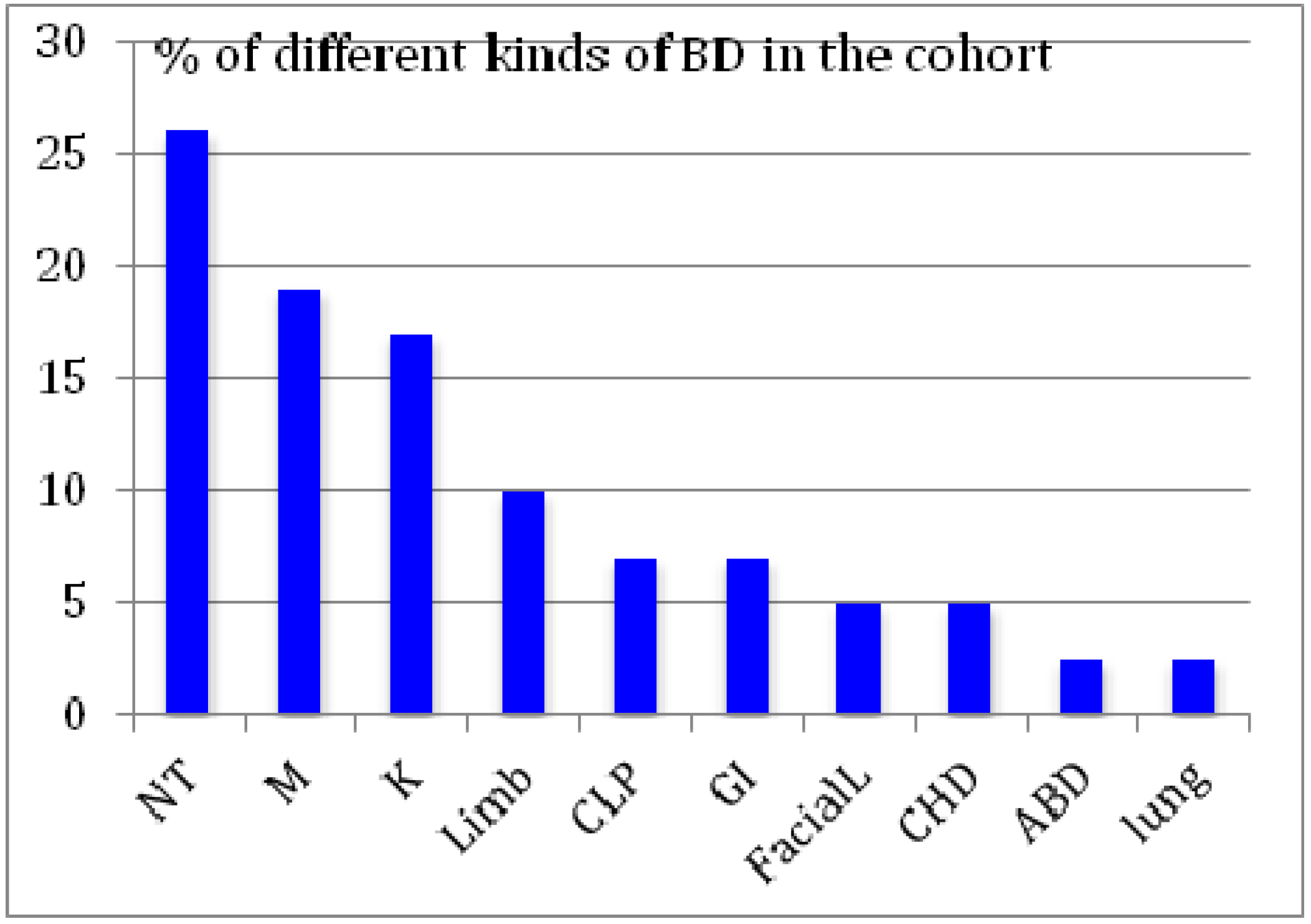
| Metal | Newborn wth BD | Normal newborn | p-value (Wilcoxon-Mann-Whitney) |
|---|---|---|---|
| Gaza 2011 (N = 48) | Gaza 2011 (N = 12) | ||
| Sn | 0.23 (0.08–0.54) | 0.04 (0.02–0.09) | 0.002 |
| Ba | 0.74 (0.51–1.27) | 0.60 (0.37–0.73) | 0.154 |
| W | 0.03 (0.02–0.07) | 0.02 (0.01–0.04) | 0.365 |
| Hg | 0.93 (0.02–0.95) | 0.00 (0.00–0.02) | 0.003 |
| Pb | 0.81 (0.49–1.16) | 0.60 (0.52–1.21) | 0.820 |
| U | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.164 |
| Se | 0.32 (0.22–0.47) | 0.13 (0.09–0.24) | 0.004 |
| Sb | 0-03 (0.02–0.06) | 0.05 (0.04–0.11) | 0.160 |
| Cd | 0-03 (0.02–0.06) | 0.05 (0.03–0.09) | 0.143 |
| Cr | 0.41 (0.29–0.59) | 0.78 (0.38–1.17) | 0.053 |
| Metal | Newborn NT defect | Newborn PCK defect | p-value (Wilcoxon-Mann-Whitney) |
|---|---|---|---|
| Gaza 2011 (N = 11) | Gaza 2011 (N = 5) | ||
| Sn | 0.32 (0.14–1.04) | 0.15 (0.06–0.30) | 0.27 |
| Ba | 0.64 (0.53–0.70) | 0.54 (0.34–0.73) | 0.66 |
| W | 0.03 (0.02–0.08) | 0.14 (0.03–0.26) | 0.28 |
| Hg | 0.05 (0.02–0.31) | 0.51 (0.17–0.95) | 0.16 |
| Pb | 1.16 (0.79–2.23) | 0.74 (0.73–1.75) | 0.66 |
| Se | 0.30 (0.22–0.69) | 0.19 (0.16–0.36) | 0.39 |
| Sb | 0.03 (0.02–0.05) | 0.04 (0.02–0.08) | 0.57 |
| Cd | 0.03 (0.02–0.07) | 0.05 (0.05–0.08) | 0.17 |
| Cr | 0.44 (0.26–0.69) | 0.47 (0.343–0.75) | 0.38 |
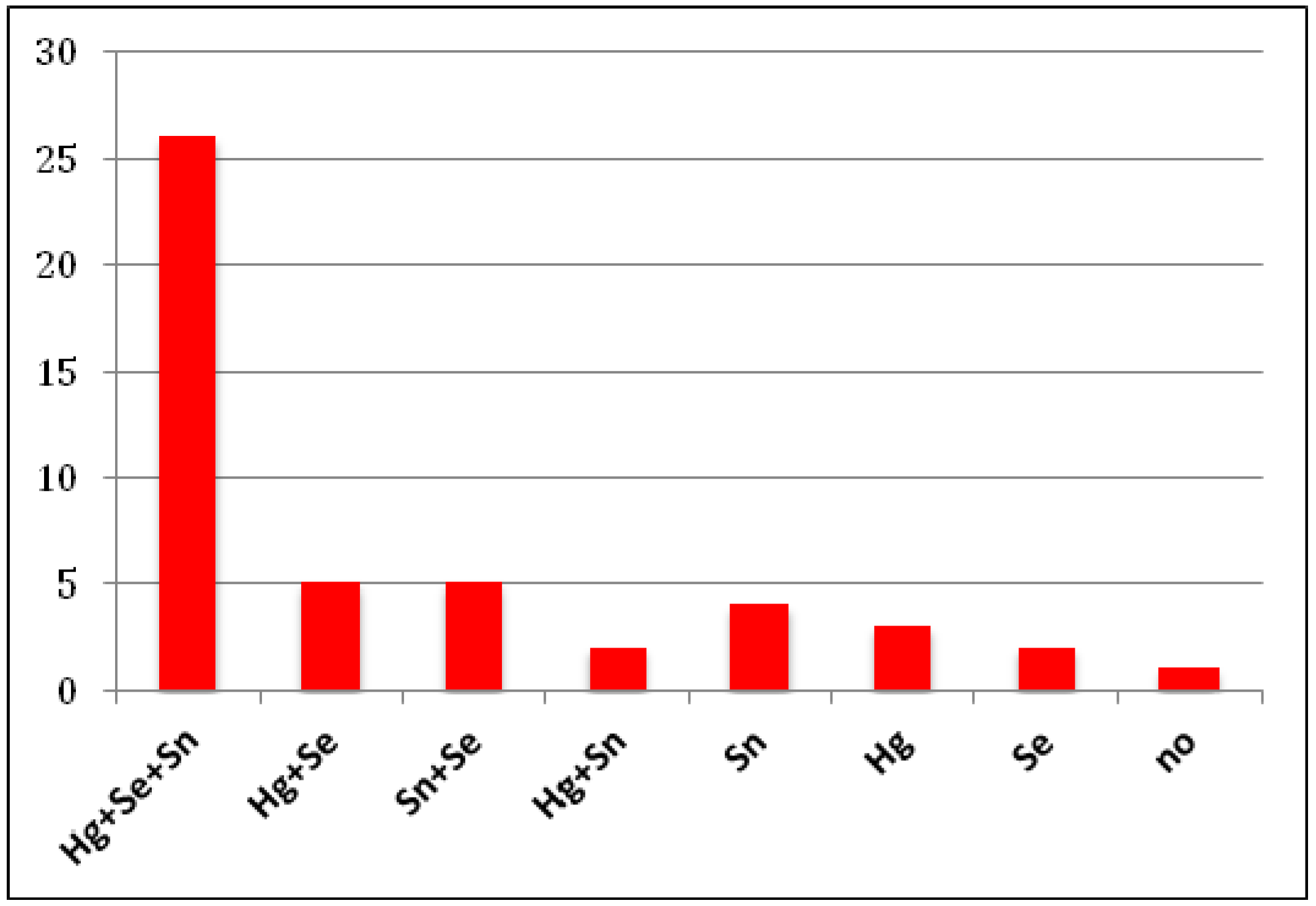
| A | |||
|---|---|---|---|
| Metal | BD exposed (N = 24) | BD not exposed (N = 13) | p-value (Wilcoxon-Mann-Witney) |
| Hg | 0.137 (0.029–0.309) | 0.087 (0.053–0.206) | 0.701 |
| Se | 0.273 (0.190–0.558) | 0.365 (0.286–0.482) | 0.364 |
| Sn | 0.262 (0.116–0.591) | 0.228 (0.049–0.535) | 0.479 |
| B | |||
| Metal | BD not exposed (N = 13) | Normal (N = 12) | p-value (Wilcoxon-Mann-Witney) |
| Hg | 0.087 (0.053–0.206) | 0.00 (0.00–0.015) | 0.005 |
| Se | 0.365 (0.286–0.482) | 0.132 (0.094–0.238) | 0.006 |
| Sn | 0.228 (0.049–0.535) | 0.042 (0.016–0.093) | 0.019 |
| Metal | Normal Newborn | Prematurely Born | |
|---|---|---|---|
| Gaza 2011 (N = 12) | Gaza 2011 (N = 9) | p-value (Wilcoxon-Mann-Whitney ) | |
| Sn | 0.04 (0.02–0.09) | 0.25 (0.23–0.89) | 0.002 |
| Ba | 0.60 (0.37–0.73) | 1.07 (0.62–1.58) | 0.03 |
| W | 0.02 (0.01–0.03) | 0.03 (0.02–0.03) | 0.19 |
| Hg | 0.00 (0.00–0.02) | 0.00 (0.00–0.05) | 0.47 |
| Pb | 0.60 (0.52–1.21) | 1.06 (0.73–2.10) | 0.19 |
| Se | 0.13 (0.09–0.24) | 0.05 (0.00–0.17) | 0.16 |
| Sb | 0.05 < 80.04–0.11) | 0.06 (0.02–0.17) | 0.55 |
| Cd | 0.05 (0.03–0.09) | 0.08 (0.06–0.09) | 0.28 |
| Cr | 0.78 (0.38–1.17) | 0.75 (0.46–0.78) | 0.81 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez-Zamudio, R.; Ha, H.C. Environmental epigenetics in metal exposure. Epigenetics 2011, 6, 820–827. [Google Scholar] [CrossRef]
- Vandegehuchte, M.B.; Janssen, C.R. Epigenetics in an ecotoxicological context. Mutat. Res. 2013, S1, 383–5718. [Google Scholar]
- Hajkova, P. Epigenetic reprogramming—Taking a lesson from the embryo. Curr. Opin. Cell Biol. 2010, 22, 342–350. [Google Scholar] [CrossRef]
- Feng, S.; Jacobsen, S.E.; Reik, W. Epigenetic reprogramming in plant and animal development. Science 2010, 330, 622–627. [Google Scholar] [CrossRef]
- Petronis, A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature 2010, 465, 721–727. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Bourc’his, D.; Voinnet, O. A small-RNA perspective on gametogenesis, fertilization, and early zygotic development. Science 2010, 330, 617–622. [Google Scholar] [CrossRef]
- Rasmussen, T.P.; Corry, G.N. Epigenetic pre-patterning and dynamics during initial stages of mammalian preimplantation development. J. Cell. Physiol. 2010, 225, 333–336. [Google Scholar] [CrossRef]
- Morgan, H.D.; Santos, F.; Green, K.; Dean, W.; Reik, W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 2005, 14, R47–R58. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 206–223. [Google Scholar] [CrossRef]
- Koedrith, P.; Kim, H.; Weon, J.I.; Seo, Y.R. Toxicogenomic approaches for understanding molecular mechanisms of heavy metal mutagenicity and carcinogenicity. Int. J. Hyg. Environ. Health 2013, 216, 587–598. [Google Scholar] [CrossRef]
- Parfitt, D.E.; Zernicka-Goetz, M. Epigenetic modification affecting expression of cell polarity and cell fate genes to regulate lineage specification in the early mouse embryo. Mol. Biol. Cell. 2010, 21, 2649–2660. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Mohideen, K.; Muhammad, R.; Davey, C.A. Perturbations in nucleosome structure from heavy metal association. Nucleic Acids Res. 2010, 38, 6301–6311. [Google Scholar] [CrossRef]
- Ong, M.S.; Vasudevan, D.; Davey, C.A. Divalent metal- and high mobility group N protein-dependent nucleosome stability and conformation. J. Nucleic Acids 2010, 2010. [Google Scholar] [CrossRef]
- Bao, Q.; Chen, H.; Liu, Y.; Yan, J.; Dröge, P.; Davey, C.A. A divalent metal-mediated switch controlling protein-induced DNA bending. J. Mol. Biol. 2007, 367, 731–740. [Google Scholar] [CrossRef]
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental Effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993, 110, 382–334. [Google Scholar]
- Cheng, T.F.; Choudhuri, S.; Muldoon-Jacobs, K. Epigenetic targets of some toxicologically relevant metals: A review of the literature. J. Appl. Toxicol. JAT 2012, 32, 643–654. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Soliman, K.F.A. Impact of environmental signals on gene expression. Epigenetics 2012, 7, 119–130. [Google Scholar] [CrossRef]
- Kim, M.; Bae, M.; Na, H.; Yang, M. Environmental toxicants—Induced epigenetic alterations and their reversers. J Environ Sci Health C. Environ. Carcinog. Ecotoxicol. Rev. 2012, 30, 323–367. [Google Scholar] [CrossRef]
- Bose, R.; Onishchenko, N.; Edoff, K.; Janson Lang, A.M.; Ceccatelli, S. Inherited effects of low-dose exposure to methylmercury in neural stem cells. Toxicol. Sci. 2012, 130, 383–390. [Google Scholar] [CrossRef]
- Dolk, H.; Vrijheid, M. The impact of environmental pollution on congenital anomalies. Br. Med. Bull. 2003, 68, 25–45. [Google Scholar] [CrossRef]
- Harada, M. Congenital Minamata disease: Intrauterine methylmercury poisoning. Teratology 1978, 18, 285–288. [Google Scholar] [CrossRef]
- Araneta, M.R.G.; Schlangen, K.M.; Edmonds, L.D.; Destiche, D.A.; Merz, R.D.; Hobbs, C.A.; Flood, T.J.; Harris, J.A.; Krishnamurti, D.; Gray, G.C. Prevalence of birth defects among infants of Gulf War veterans in Arkansas, Arizona, California, Georgia, Hawaii, and Iowa, 1989–1993. Birth Defects Res. A 2003, 67, 246–260. [Google Scholar] [CrossRef]
- Vidosavljević, D.; Puntarić, D.; Gvozdić, V.; Jergović, M.; Miškulin, M.; Puntarić, I.; Puntarić, E.; Šijanović, S. Soil contamination as a possible long-term consequence of war in Croatia. Acta Agric. Scand. Section B Soil Plant Sci. 2013, 63, 322–329. [Google Scholar]
- Jergović, M.; Miškulin, M.; Puntarić, D.; Gmajnić, R.; Milas, J.; Sipos, L. Cross-sectional biomonitoring of metals in adult populations in post-war eastern Croatia: Differences between areas of moderate and heavy Combat. Croat Med. J. 2010, 51, 451–460. [Google Scholar] [CrossRef]
- Skaik, S.; Abu-Shaban, N.; Abu-Shaban, N.; Barbieri, M.; Barbieri, M.; Giani, U.; Manduca, P. Metals detected by ICP/MS in wound tissue of war injuries without fragments in Gaza. BMC Int. Health Hum. Rights 2010, 10, 17–25. [Google Scholar] [CrossRef]
- Manduca, P.; Barbieri, M.; Barbieri, M. Craters Datasheet Results. 2009. Available online: http://www.newweapons.org/?q=node/110 (accessed on 24 February 2014).
- O’Reilly, J.; Shin, J. BAE Systems Armament Systems Division. Available online: http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA476392Minneapolis (accessed on 24 February 2014).
- Manduca, P.; Barbieri, M.; Barbieri, M. WP Bomb Datasheet Results. 2009. Available online: http://www.newweapons.org/?q=node/110 (accessed on 24 February 2014).
- Alaani, S.; Tafash, M.; Busby, C.; Hamdan, M.; Blaurock-Busch, E. Uranium and other contaminants in hair from the parents of children with congenital anomalies in Fallujah, Iraq. Confl. Health 2011, 5, 15–20. [Google Scholar] [CrossRef]
- Manduca, P. High Prevalence Data and Increase in Time of Birth Defects in Fallujah, Iraq: Historical Reproductive Life and Hair Metal Load in Newborns and Children with Birth Defects and Their Families. Available online: http://www.newweapons.org/?q=node/120 (accessed on 24 February 2014).
- Manduca, P.; Barbieri, M.; Barbieri, M. Metals Detected in Palestinian Children’s Hair Suggest Environmental Contamination. Available online: http://www.newweapons.org/?q=node/112 (accessed on 24 February 2014).
- Naim, A.; Al Dalies, H.; El Balawi, M.; Salem, E.; Al Meziny, K.; Al Shawwa, R.; Minutolo, R.; Manduca, P. Structural birth defects in the Gaza Strip, occupied Palestinian territory: A cohort study. Lancet 2013, 380, S30–S31. [Google Scholar]
- Naim, A.; Al Dalies, H.; El Balawi, M.; Salem, E.; Al Meziny, K.; Al Shawwa, R.; Minutolo, R.; Manduca, P. Birth defects in Gaza: Prevalence, types, familiarity and correlation with environmental factors. Int. J. Environ. Res. Public Health 2012, 9, 1732–1747. [Google Scholar] [CrossRef]
- International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: http://apps.who.int/classifications/icd10/browse/2010/en (accessed on 24 February 2014).
- Arai, Y.; Ohgane, J.; Yagi, S.; Ito, R.; Iwasaki, Y.; Saito, K.; Akutsu, K.; Takatori, S.; Ishii, R.; Hayashi, R.; et al. Epigenetic assessment of environmental chemicals detected in maternal peripheral and cord blood samples. J. Reprod. Dev. 2011, 57, 507–517. [Google Scholar] [CrossRef]
- Vahter, M.; Akesson, A.; Lind, B.; Bjors, U.; Schutz, A.; Berglund, M. Longitudinal study of methylmercury and inorganic mercury in blood and urine of pregnant and lactating women, as well as in umbilical cord blood. Environ. Res. 2000, 84, 186–194. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Shinwari, N.; Mashhour, A.; Mohamed Gel, D.; Rabah, A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int. J. Hyg. Environ. Health 2011, 214, 79–101. [Google Scholar] [CrossRef]
- Sakamoto, M.; Yasutake, A.; Domingo, J.L.; Chan, H.M.; Kubota, M.; Murata, K. Relationships between trace element concentrations in chorionic tissue of placenta and umbilical cord tissue: Potential use as indicators for prenatal exposure. Environ. Int. 2013, 60, 106–111. [Google Scholar]
- Skinner, M.K.; Haque, C.G.; Nilsson, E.; Bhandari, R.; McCarrey, J.R. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Alattia, J.R.; Kuraishi, T.; Dimitrov, M.; Chang, I.; Lemaitre, B.; Fraering, P.C. Mercury is a direct and potent γ-secretase inhibitor affecting Notch processing and development in Drosophila. FASEB J. 2011, 25, 2287–2295. [Google Scholar] [CrossRef]
- Abnoos, H.; Fereidoni, M.; Mahdavi-Shahri, N.; Haddad, F.; Jalal, R. Developmental study ofmercuryeffects on the fruit fly (Drosophila melanogaster). Interdiscip. Toxicol. 2013, 6, 34–40. [Google Scholar]
- Wu, Q.; He, K.; Liu, P.; Li, Y.; Wang, D. Association of oxidative stress with the formation of reproductive toxicity from mercury exposure on hermaphrodite nematode Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2011, 32, 175–184. [Google Scholar]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Fossato da Silva, D.A.; Teixeira, C.T.; Scarano, W.R.; Favareto, A.P.; Fernandez, C.D.; Grotto, D.; Barbosa, F., Jr.; Kempinas Wde, G. Effects of methylmercury on male reproductive functions in Wistar rats. Reprod. Toxicol. 2011, 31, 431–439. [Google Scholar]
- Abd El-Aziz, G.S.; El-Fark, M.M.; Saleh, H.A. The prenatal toxic effect of methylmercury on the development of the appendicular skeleton of rat fetuses and the protective role of vitamin E. Anat. Rec. (Hoboken). 2012, 295, 939–949. [Google Scholar] [CrossRef]
- Cace, I.B.; Milardovic, A.; Prpic, I.; Krajina, R.; Petrovic, O.; Vukelic, P.; Spiric, Z.; Horvat, M.; Mazej, D.; Snoj, J. Relationship between the prenatal exposure to low-level of mercury and the size of a newborn’s cerebellum. Med. Hypotheses 2011, 76, 514–516. [Google Scholar] [CrossRef]
- Pohl, H.R.; Roney, N.; Abadin, H.G. Metal ions affecting the neurological system. Met. Ions Life Sci. 2011, 8, 247–262. [Google Scholar]
- Johansson, C.; Castoldi, A.F.; Onishchenko, N.; Manzo, L.; Vahter, M.; Ceccatelli, S. Neurobehavioural and molecular changes induced by methylmercury exposure during development. Neurotox Res. 2007, 11, 241–260. [Google Scholar] [CrossRef]
- Parran, D.K.; Barone, S., Jr.; Mundy, W.R. Methylmercury decreases NGF-induced TrkA autophosphorylation and neurite outgrowth in PC12 cells. Brain Res. Dev. Brain Res. 2003, 141, 71–81. [Google Scholar] [CrossRef]
- Larkfors, L.; Oskarsson, A.; Sundberg, J.; Ebendal, T. Methylmercury induced alterations in the nerve growth factor level in the developing brain. Brain Res. Dev. Brain Res. 1991, 62, 287–291. [Google Scholar] [CrossRef]
- Onishchenko, N.; Karpova, N.; Sabri, F.; Castren, E.; Ceccatelli, S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J. Neurochem. 2008, 106, 1378–1387. [Google Scholar] [CrossRef]
- Ceccatelli, S.; Bose, R.; Edoff, K.; Onishchenko, N.; Spulber, S. Long-lasting neurotoxic effects of exposure to methylmercury during development. J. Intern. Med. 2013, 273, 490–497. [Google Scholar] [CrossRef]
- Vinceti, M.; Crespi, C.M.; Bonvicini, F.; Malagoli, C.; Ferrante, M.; Marmiroli, S.; Stranges, S. The need for a reassessment of the safe upper limit of selenium in drinking water. Sci. Total Environ. 2013, 443, 633–642. [Google Scholar] [CrossRef]
- Beyrouty, P.; Chan, H.M. Co-consumption of selenium and vitamin E altered the reproductive and developmental toxicity of methylmercury in rats. Neurotoxicol. Teratol. 2006, 28, 49–58. [Google Scholar] [CrossRef]
- Florea, A.M.; BÅNusselberg, D. Metals and breast cancer: Risk factors or healing agents? J. Toxicol. 2011, 2011. [Google Scholar] [CrossRef]
- Hoffman, D.J. Role of selenium toxicity and oxidative stress in aquatic birds. Aquat. Toxicol. 2002, 57, 11–26. [Google Scholar] [CrossRef]
- Usami, M.; Mitsunaga, K.; Nakazawa, K.; Doi, O. Proteomic analysis of selenium embryotoxicity in cultured postimplantation rat embryos. Birth Defects Res. B Dev. Reprod. Toxicol. 2008, 83, 80–96. [Google Scholar] [CrossRef]
- Heinz, G.H.; Hoffman, D.J.; Klimstra, J.D.; Stebbins, K.R. A comparison of the teratogenicity of methylmercury and selenomethionine injected into bird eggs. Arch. Environ. Contam Toxicol. 2012, 62, 519–528. [Google Scholar] [CrossRef]
- Moser, V.C.; McGee, J.K.; Ehman, K.D. Concentration and persistence of tin in rat brain and blood following dibutyltin exposure during development. J. Toxicol. Environ. Health A. 2009, 72, 47–52. [Google Scholar] [CrossRef]
- Relevance to Public Health. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp24-c2.pdf (accessed on 24 February 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Manduca, P.; Naim, A.; Signoriello, S. Specific Association of Teratogen and Toxicant Metals in Hair of Newborns with Congenital Birth Defects or Developmentally Premature Birth in a Cohort of Couples with Documented Parental Exposure to Military Attacks: Observational Study at Al Shifa Hospital, Gaza, Palestine. Int. J. Environ. Res. Public Health 2014, 11, 5208-5223. https://doi.org/10.3390/ijerph110505208
Manduca P, Naim A, Signoriello S. Specific Association of Teratogen and Toxicant Metals in Hair of Newborns with Congenital Birth Defects or Developmentally Premature Birth in a Cohort of Couples with Documented Parental Exposure to Military Attacks: Observational Study at Al Shifa Hospital, Gaza, Palestine. International Journal of Environmental Research and Public Health. 2014; 11(5):5208-5223. https://doi.org/10.3390/ijerph110505208
Chicago/Turabian StyleManduca, Paola, Awny Naim, and Simona Signoriello. 2014. "Specific Association of Teratogen and Toxicant Metals in Hair of Newborns with Congenital Birth Defects or Developmentally Premature Birth in a Cohort of Couples with Documented Parental Exposure to Military Attacks: Observational Study at Al Shifa Hospital, Gaza, Palestine" International Journal of Environmental Research and Public Health 11, no. 5: 5208-5223. https://doi.org/10.3390/ijerph110505208





