A Seventeen-Year Epidemiological Surveillance Study of Borrelia burgdorferi Infections in Two Provinces of Northern Spain
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Serum Samples
2.3. Immunofluorescence

2.4. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis and Western Blotting
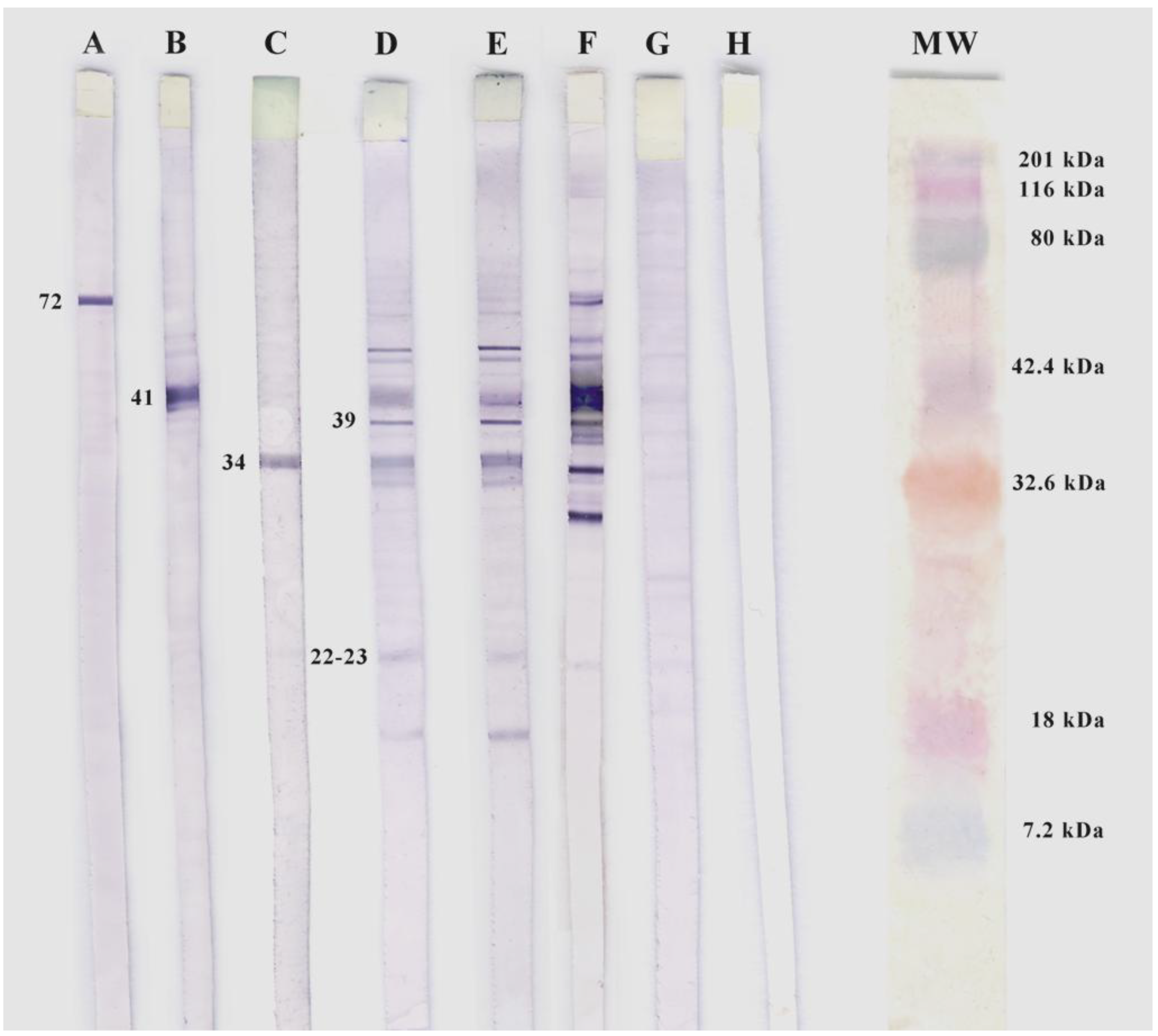
2.5. Analysis of Ticks for Borrelia burgdorferi DNA
2.6. Statistical Analysis
3. Results
| Age groups | Number (%) |
|---|---|
| 0–10 years | 8 (9.2) |
| 11–20 years | 10 (17.2) |
| 21–30 years | 5 (11.3) |
| 31–40 years | 14 (15.38) |
| 41–50 years | 19 (16.9) |
| 51–60 years | 7 (8.1) |
| 61–70 years | 10 (13.7) |
| 71–80 years | 8 (13.8) |
| 81–90 years | 2 (15.38) |
| 91–more years | ND |
| Patients occupation | Number (%) |
|---|---|
| Farmer | 5 (7.9) |
| Livestock | 3 (9.6) |
| Pastors | 1 (7.7) |
| Construction workers | 6 (25) |
| Administrative workers (clerks, lawyers, business) | 4 (14.2) |
| Service sector (shopkeepers, waiters, cooks, mechanics, maintenance) | 8 (25) |
| Veterinarians | 1 (33.3) |
| Health (doctors and nurses) | 2 (20%) |
| Houesewives | 13 (12.6) |
| Retirees | 10 (14.3) |
| Students | 5 (7.35) |
| Unspecified profession (mostly children under five years) | 25 (18.38) |
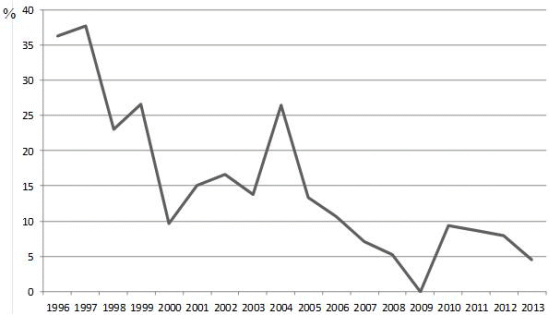
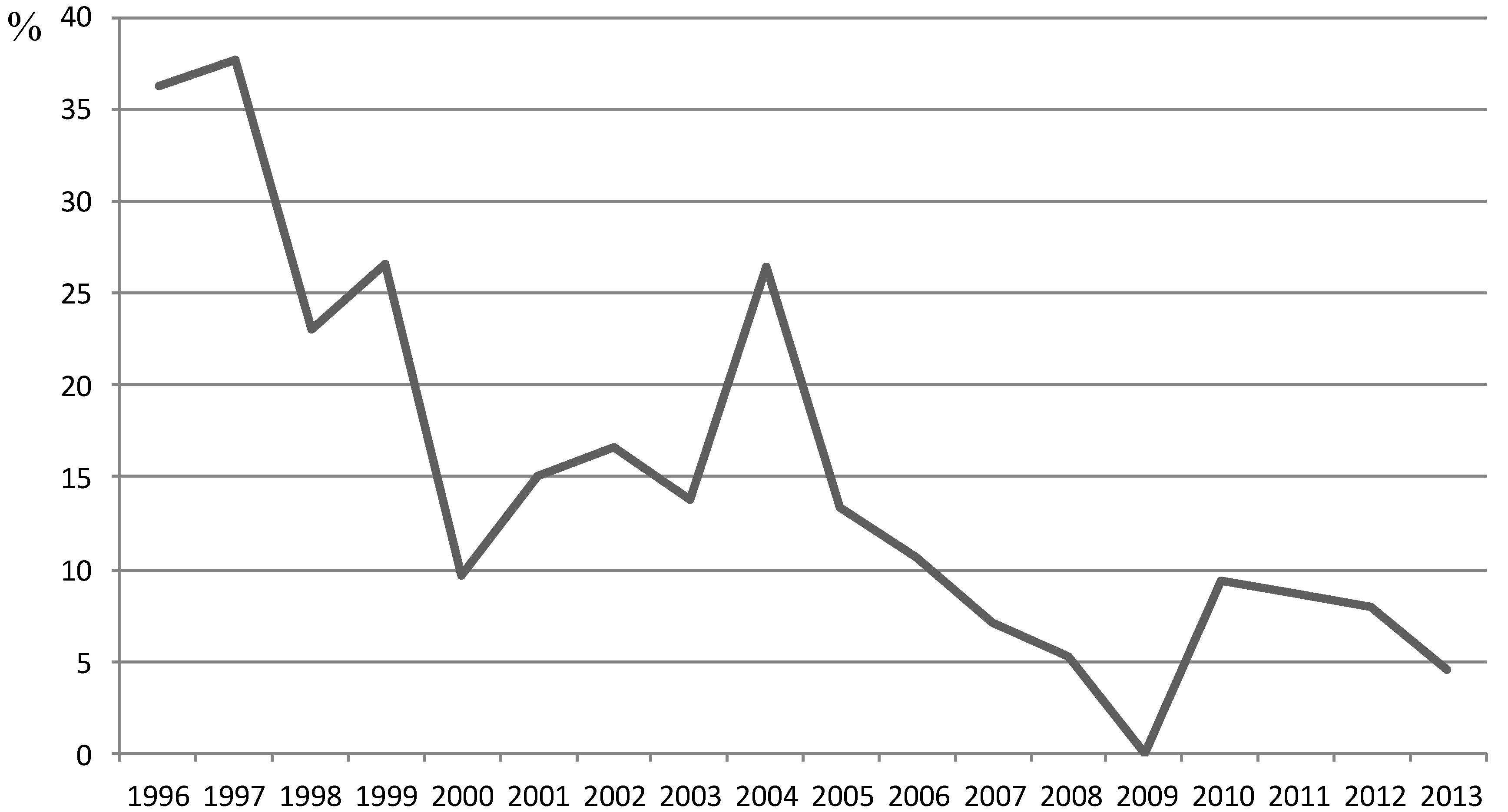
| Years | Ig G prevalence (%) | IgM prevalence (%) | IgG+IgM prevalence (%) |
|---|---|---|---|
| 1996 | 18.18 | 27.27 | 9.09 |
| 1997 | 15.55 | 35.55 | 15.55 |
| 1998 | 2.56 | 23.07 | 2.56 |
| 1999 | 20 | 13.33 | 6.66 |
| 2000 | 9.67 | 6.45 | 6.45 |
| 2001 | 15 | 10 | 10 |
| 2002 | 16.66 | 16.66 | 16.66 |
| 2003 | 13.79 | 3.44 | 3.44 |
| 2004 | 26.47 | 14.7 | 14.7 |
| 2005 | 10 | 6.66 | 3.33 |
| 2006 | 10.71 | 0 | 0 |
| 2007 | 7.14 | 0 | 0 |
| 2008 | 5.26 | 1.74 | 1.74 |
| 2009 | 0 | 0 | 0 |
| 2010 | 9.3 | 2.32 | 2.32 |
| 2011 | 6.89 | 3.44 | 1.74 |
| 2012 | 7.89 | 0 | 0 |
| 2013 | 4.54 | 0 | 0 |
4. Discussion and Conclusions
Acknowledgments
Conflicts of Interest
References
- Lindgren, E.; Tälleklint, L.; Polfeldt, T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 2000, 108, 119–123. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ortega, C.; Sánchez, N.; Desimone, L.; Sudre, B.; Suk, J.E.; Semenza, J.C. Correlation of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks with specific abiotic traits in the western palearctic. Appl. Environ. Microbiol. 2011, 77, 3838–3845. [Google Scholar] [CrossRef]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex—clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme disease—a tick-borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar]
- Dantas-Torres, F.; Otranto, D. Species diversity and abundance of ticks in three habitats in southern Italy. Ticks Tick-Borne Dis. 2013, 4, 251–255. [Google Scholar] [CrossRef]
- García-Moncó, J.C.; Benach, J.L.; Coleman, J.L.; Galbe, J.L.; Szczepanski, A.; Fernández-Villar, B.; Norton-Hughes, C.A.; Johnson, R.C. The characterization of a Spanish strain of Borrelia burgdorferi. Med. Clin. (Barc) 1992, 98, 89–93. [Google Scholar]
- Oteo, J.A.; Martínez de Artola, V.; Gómez-Cadiñanos, R.; Casas, J.M.; Blanco, J.R.; Rosel, L. Evaluation of methods of tick removal in human ixodidiasis. Rev. Clin. Esp. 1996, 196, 584–587. [Google Scholar]
- Lledó, L.; Gegúndez, M.I.; Saz, J.V.; Beltrán, M. Screening of the prevalence of antibodies to Borrelia burgdorferi in Madrid province, Spain. Eur. J. Epidemiol. 2004, 19, 471–472. [Google Scholar]
- Márquez-Jiménez, F.J.; Hidalgo-Pontiveros, A.; Contreras-Chova, F.; Rodríguez-Liébana, J.J; Muniain-Ezcurra, M.A. Ticks (Acarina: Ixodidae) as vectors and reservoirs of pathogen microorganisms in Spain. Enferm. Infecc. Microbiol. Clin. 2005, 23, 94–102. [Google Scholar] [CrossRef]
- Anda, P.; Rodríguez, I.; de la Loma, A.; Fernández, M.V.; Lozano, A. A serological survey and review of clinical Lyme borreliosis in Spain. Clin. Infect. Dis. 1993, 16, 310–319. [Google Scholar] [CrossRef]
- Guerrero, A.; Escudero, R.; Marti-Belda, P.; Quereda, C. Frecuencia de las manifestaciones clínicas de la borreliosis de Lyme en España. Enferm. Infecc. Microbiol. Clin. 1996, 14, 72–79. [Google Scholar]
- Gutiérrez, J.; Guerrero, M.; Núñez, F.; Soto, M.J.; Piédrola, G.; del Carmen Maroto, M. Antibodies to Borrelia burgdorferi in European populations. J. Clin. Lab. Anal. 2000, 14, 20–26. [Google Scholar] [CrossRef]
- Escudero-Nieto, R.; Guerrero-Espejo, A. Diseases produced by Borrelia. Enferm. Infecc. Microbiol. Clin. 2005, 23, 232–240. [Google Scholar] [CrossRef]
- Oteo, J.A.; Martínez de Artola, V.; Fernández-Calvo, J.L.; Casas, J.M.; Rivero, A.; Grandival, R. The prevalence of Borrelia burgdorferi antibodies in a population at risk. Rev. Clin. Esp. 1990, 187, 215–217. [Google Scholar]
- Arteaga, F.; García-Moncó, J.C. Association of Lyme disease with work and leisure activities. Enferm. Infecc. Microbiol. Clin. 1998, 16, 265–268. [Google Scholar]
- Anda, P.; Sánchez-Yebra, W.; del Mar Vitutia, M.; Pérez-Pastrana, E.; Rodríguez, I.; Miller, N.S.; Backenson, P.B.; Benach, J.L. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet 1996, 348, 162–165. [Google Scholar] [CrossRef]
- Aislamiento de Borrelia garinii a partir de un paciente (eritema migrans). Available online: http://www.seimc.org (accessed on 29 January 2014).
- Ma, B.; Christen, B.; Leung, D.; Vigo-Pelfrey, C. Serodiagnosis of Lyme borreliosis by western immunoblot: Reactivity of various significant antibodies against Borrelia burgdorferi. J. Clin. Microbiol. 1992, 30, 370–376. [Google Scholar]
- Guttman, D.S.; Wang, P.W.; Wang, I.N.; Bosler, E.M.; Luft, B.J.; Dykhuizen, D.E. Multiple infections of Ixodes scapularis ticks by Borrelia burgdorferi as revealed by single-strand conformation polymorphism analysis. J. Clin. Microbiol. 1996, 34, 652–656. [Google Scholar]
- Postic, D.; Assous, M.V.; Grimont, P.A.D.; Baranton, G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int. J. Syst. Evolut. Microbiol. 1994, 44, 743–752. [Google Scholar]
- Guerrero-Espejo, A. Lyme disease in Spain. Med. Clin. (Barc) 1992, 98, 96–97. [Google Scholar]
- Vorou, R.M.; Papavassiliou, V.G.; Tsiodras, S. Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol. Infect. 2007, 135, 1231–1247. [Google Scholar]
- Estrada-Peña, A.; Ayllón, N.; de la Fuente, J. Impact of climate trends on tick-borne pathogen transmission. Front Physiol. 2012, 3. [Google Scholar] [CrossRef]
- López-Prieto, M.D.; Borobio, M.V. Prevalence of antibodies against Borrelia burgdorferi among the population of Seville. Enferm. Infecc. Microbiol. Clin. 1989, 7, 489–490. [Google Scholar]
- Tamayo, L.; García-Moncó, J.C.; Bratos, M.A.; Orduña, A.; Ortiz de Lejarazu, R.; Rodríguez-Torres, A. Antibodies against Borrelia burgdorferi in a population group from Valladolid. Enferm. Infecc. Microbiol. Clin. 1990, 8, 663–664. [Google Scholar]
- Oteo-Revuelta, J.A.; Martínez de Artola, V. Lyme borreliosis: Epidemiologic and etiopathogenic aspects. Enferm. Infecc. Microbiol. Clin. 1995, 13, 550–555. [Google Scholar]
- Oteiza-Olaso, J.; Tiberio-López, G.; Martínez de Artola, V.; Belzunegui-Otano, T. Seroprevalence of Lyme disease in Navarra, Spain. Med. Clin. (Barc) 2011, 26, 336–339. [Google Scholar]
- Nohlmans, M.K.; van den Bogaard, A.E.; Blauw, A.A.; van Boven, C.P. Prevalence of Lyme borreliosis in The Netherlands. Ned. Tijdschr. Geneeskd. 1991, 135, 2288–2292. [Google Scholar]
- Calderaro, A.; Montecchini, S.; Gorrini, C.; Piccolo, G.; Chezzi, C.; Dettori, G. Presence of anti-Borrelia burgdorferi antibodies and Borrelia burgdorferi sensu lato DNA in samples of subjects in an area of the Northern Italy in the period 2002–2008. Diagn. Microbiol. Infect. Dis. 2011, 70, 455–460. [Google Scholar] [CrossRef]
- Arteaga-Pérez, F.; García-Moncó, J.C. Risk factors associated with the presence of antibodies against Borrelia burgdorferi. Rev. Clin. Esp. 1999, 199, 136–141. [Google Scholar]
- Buczek, A.; Rudek, A.; Bartosik, K.; Szymanska, J.; Wojcik-Fatla, A. Seroepidemiological study of Lyme borreliosis among forestry workers in southern Poland. Ann. Agric. Environ. Med. 2009, 16, 257–261. [Google Scholar]
- Kubiak, K.; Dzika, E.; Równiak, J.; Dziedziech, M.; Dzisko, J. Seroprevalence of Lyme disease and genospecies of Borrelia burgdorferi sensu lato in patients diagnosed with borreliosis in the Province of Warmia-Masuria in north-eastern Poland. Ann. Agric. Environ. Med. 2012, 19, 203–207. [Google Scholar]
- Chmielewska-Badora, J.; Moniuszko, A.; Żukiewicz-Sobczak, W.; Zwoliński, J.; Piątek, J.; Pancewicz, S. Serological survey in persons occupationally exposed to tick-borne pathogens in cases of co-infections with Borrelia burgdorferi, Anaplasma phagocytophilum, Bartonella spp. and Babesia microti. Ann. Agric. Environ. Med. 2012, 19, 271–274. [Google Scholar]
- Dehnert, M.; Fingerle, V.; Klier, C.; Talaska, T.; Schlaud, M.; Krause, G.; Wilking, H.; Poggensee, G. Seropositivity of Lyme borreliosis and associated risk factors: A population-based study in Children and Adolescents in Germany (KiGGS). PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Gerber, M.A.; Shapiro, E.D.; Burke, G.S.; Parcells, V.J.; Bel, G.L.; for the Pediatric Lyme Disease Study Group. Lyme disease in children in southeastern Connecticut. N. Eng. J. Med. 1996, 335, 1270–1274. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Núñez, F.; Utilla, N.; Maroto, M.C. Borreliosis de Lyme en el niño: doble infección o evolución atípica. Med. Clin. (Barc) 1995, 105, 317–318. [Google Scholar]
- Gajovic, O.; Todorovic, Z.; Nesic, L.; Lazic, Z. Lyme borreliosis—diagnostic difficulties in interpreting serological results. Med. Pregl. 2010, 63, 839–843. [Google Scholar] [CrossRef]
- Rahn, D.W. Lyme disease: Clinical manifestations, diagnosis and treatment. Semin. Arthritis Reum. 1991, 20, 201–218. [Google Scholar] [CrossRef]
- Roca, B. Lyme borreliosis. Med. Clin. (Barc) 2006, 127, 265–268. [Google Scholar] [CrossRef]
- Campbell, G.L.; Fritz, C.; Fish, D.; Nowakowski, J.; Nadelman, R.B.; Wormser, G.P. Estimation of the incidence of Lyme disease. Am. J. Epidemiol. 1998, 148, 1018–1026. [Google Scholar] [CrossRef]
- Oteo-Revuelta, J.A.; Estrada-Peña, A. Ixodes ricinus, a demonstrated vector of Borrelia burgdorferi in Spain. Med. Clin. (Barc) 1991, 96, 599. [Google Scholar]
- Meiners, T.; Hammer, B.; Göbel, U.B.; Kahl, O. Determining the tick scutal index allows assessment of tick feeding duration and estimation of infection risk with Borrelia burgdorferi sensu lato in a person bitten by an Ixodes ricinus nymph. Int. J. Med. Microbiol. 2006, 296, 103–107. [Google Scholar]
- Kempf, F.; de Meeûs, T.; Arnathau, C.; Degeilh, B.; McCoy, K.D. Assortativ pairing in Ixodes ricinus (Acari: Ixodidae), the European vector of Lyme borreliosis. J. Med. Entomol. 2009, 46, 471–474. [Google Scholar] [CrossRef]
- Bonnet, S.; de la Fuente, J.; Nicollet, P.; Liu, X.; Madani, N.; Blanchard, B.; Maingourd, C.; Alongi, A.; Torina, A.; Fernández de Mera, I.G.; et al. Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector-Borne Zoonotic Dis. 2013, 13, 226–236. [Google Scholar] [CrossRef]
- Reye, A.L.; Stegniy, V.; Mishaeva, N.P.; Velhin, S.; Hübschen, J.M.; Ignatyev, G.; Muller, C.P. Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Karbowiak, G.; Kiewra, D. New locations of Dermacentor reticulatus ticks in Western Poland: The first evidence of the merge in D. reticulatus occurrence areas? Wiad. Parazytol. 2010, 56, 333–336. [Google Scholar]
- Tomanović, S.; Chochlakis, D.; Radulović, Z.; Milutinović, M.; Cakić, S.; Mihaljica, D.; Tselentis, Y.; Psaroulaki, A. Analysis of pathogen co-occurrence in host-seeking adult hard ticks from Serbia. Exp. Appl. Acarol. 2013, 59, 367–376. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lledó, L.; Gegúndez, M.I.; Giménez-Pardo, C.; Álamo, R.; Fernández-Soto, P.; Nuncio, M.S.; Saz, J.V. A Seventeen-Year Epidemiological Surveillance Study of Borrelia burgdorferi Infections in Two Provinces of Northern Spain. Int. J. Environ. Res. Public Health 2014, 11, 1661-1672. https://doi.org/10.3390/ijerph110201661
Lledó L, Gegúndez MI, Giménez-Pardo C, Álamo R, Fernández-Soto P, Nuncio MS, Saz JV. A Seventeen-Year Epidemiological Surveillance Study of Borrelia burgdorferi Infections in Two Provinces of Northern Spain. International Journal of Environmental Research and Public Health. 2014; 11(2):1661-1672. https://doi.org/10.3390/ijerph110201661
Chicago/Turabian StyleLledó, Lourdes, María Isabel Gegúndez, Consuelo Giménez-Pardo, Rufino Álamo, Pedro Fernández-Soto, María Sofia Nuncio, and José Vicente Saz. 2014. "A Seventeen-Year Epidemiological Surveillance Study of Borrelia burgdorferi Infections in Two Provinces of Northern Spain" International Journal of Environmental Research and Public Health 11, no. 2: 1661-1672. https://doi.org/10.3390/ijerph110201661