Suitability of Optical, Physical and Chemical Measurements for Detection of Changes in Bacterial Drinking Water Quality
Abstract
:1. Introduction
2. Material and Methods
2.1. Monitoring Growth of Pseudomonas fluorescens
2.2. Monitoring Escherichia coli in Spring Water
2.3. Monitoring of Water Samples from Distribution Systems
2.4. The Case of an Accidentally Contaminated Distribution Network
2.5. Statistical Analysis
3. Results
| N | Median | Min–Max | |
|---|---|---|---|
| Pseudomonas fluorescens | |||
| colony counts | 74 | 9.3 × 105 | 1.0 × 102–8.8 × 106 |
| total cell counts | 58 | 8.9 × 105 | 1.9 ×102–4.6 × 107 |
| E. coli colony counts | 20 | 3.05 × 104 | 0–3.6 × 107 |
3.1. Growth of Pseudomonas fluorescens
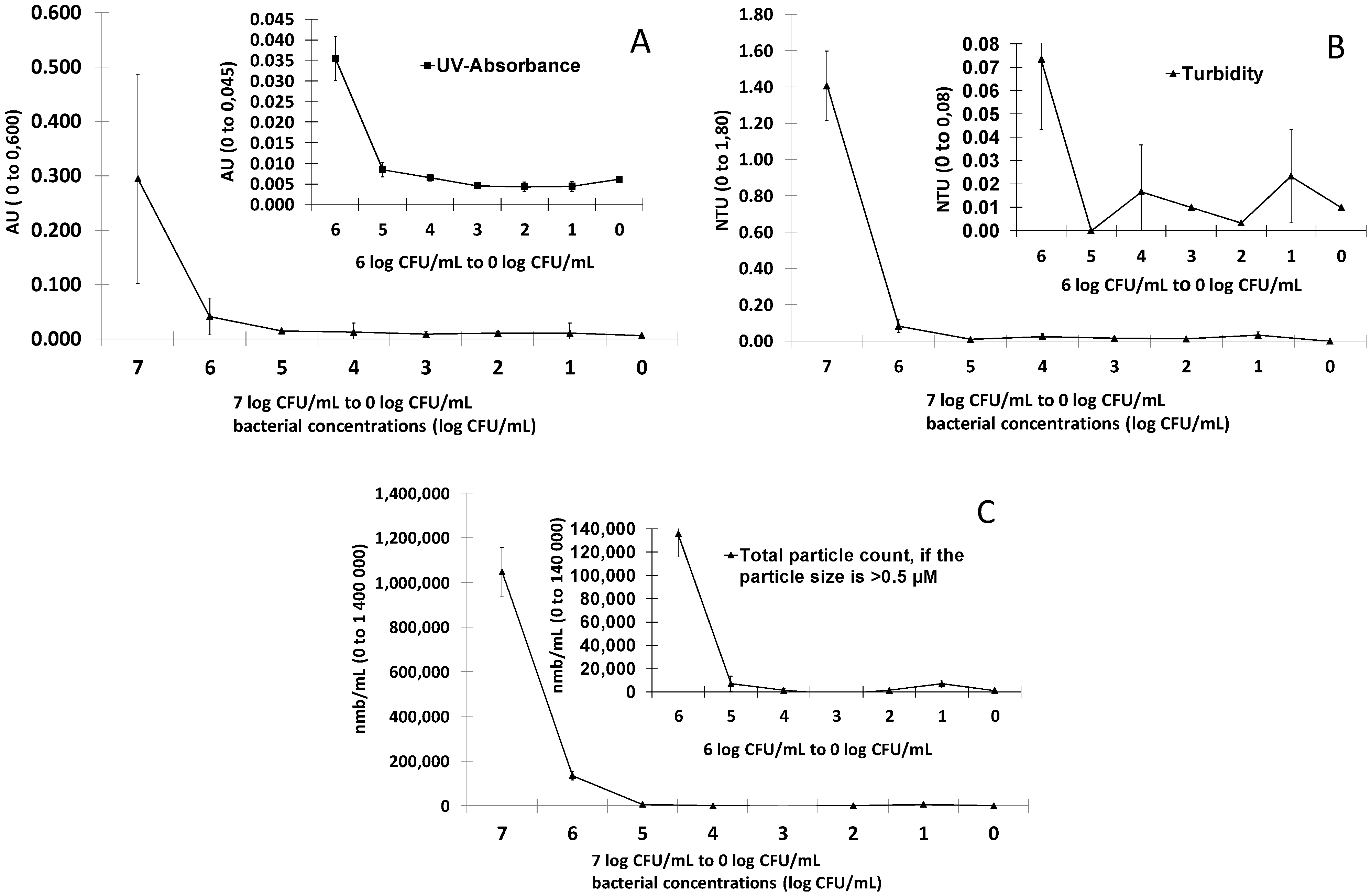
3.2. Detection of E. coli in Spring Water
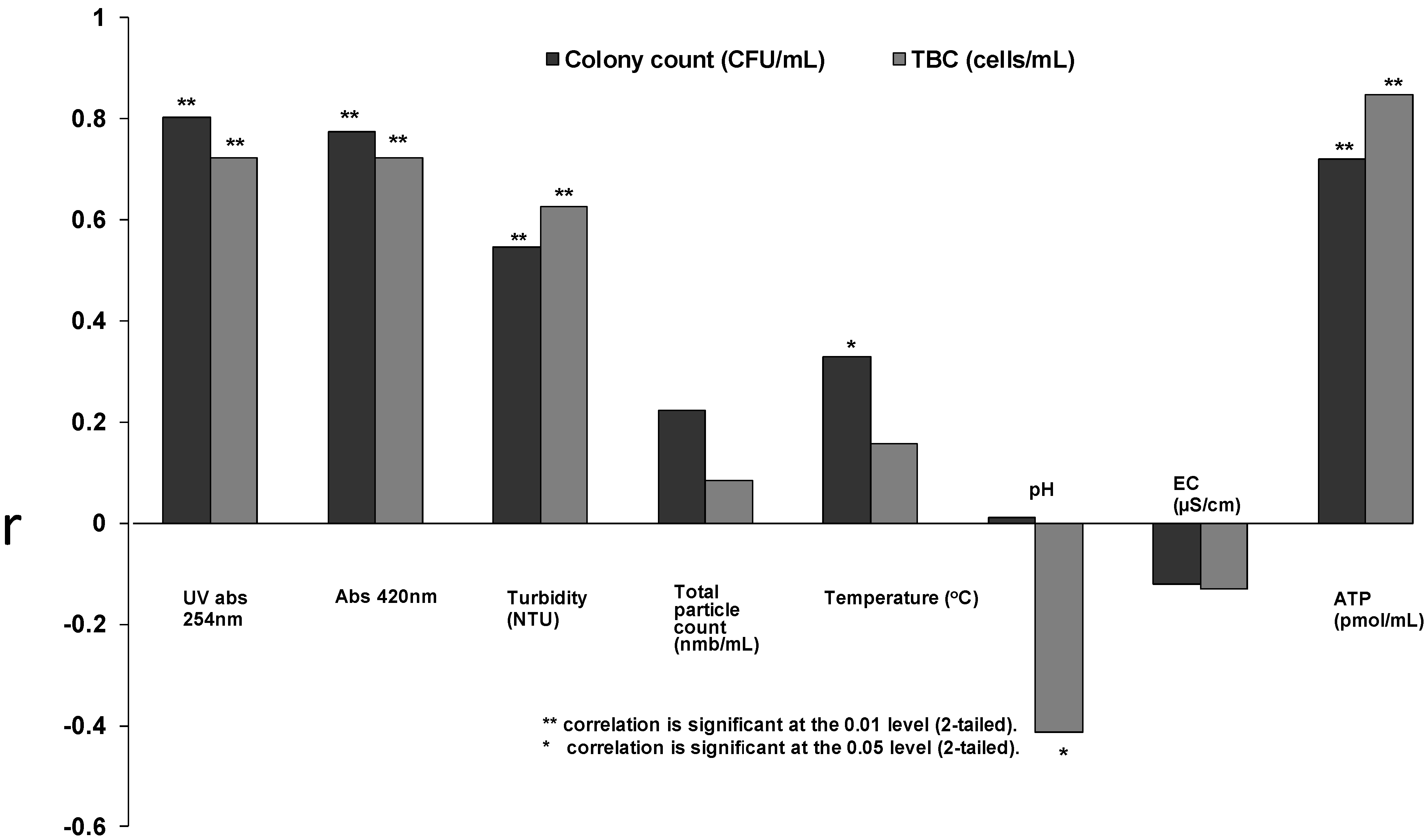
3.3. Monitoring of the Distribution System Water Samples
| N | Median | Min–Max | |
|---|---|---|---|
| Optical | |||
| Abs 420 | 52 | 0.01 | 0.001–0.330 |
| UV Abs 254 | 52 | 0.133 | 0.019–0.416 |
| Turbidity (NTU) | 52 | 0.257 | 0.04–27.4 |
| Total particle count (nmb/mL) | 51 | 1,718 | 1.1 × 102–6.1 × 104 |
| Physical | |||
| Temperature (°C) | 52 | 7.7 | 5.1–21.0 |
| pH | 52 | 6.905 | 6.40–8.78 |
| EC(µS/cm) | 52 | 249 | 18–475 |
| Chemical | |||
| Free chlorine (mg/L) | 18 | 0.225 | 0.10–0.64 |
| ATP (pmol/mL) | 52 | 41 | 0.04–119 |
| Bacterial | |||
| HPC on R2A (CFU/mL) | 48 | 656 | 10–1.3 × 105 |
| HPC on YEA (CFU/mL) | 51 | 3 | 0–5.3 × 103 |
| TBC (cells/mL) | 52 | 55,784 | 1.1 × 103–7.8 × 105 |
| Coliforms (CFU/1,000 mL) | 34 | BDL1 | BDL1 |
| Enterococci (CFU/1,000 mL) | 34 | BDL | BDL |
| Nutrients | |||
| AOC-C/L µg | 14 | 128 | 60.9–179.1 |
| TOC mg/L | 14 | 3 | 1.6–3.3 |
| MAP µg/L | 13 | 0.0085 | 0.0007–0.1533 |
| Total-P µg/L | 14 | 1.85 | 0.6–3.0 |
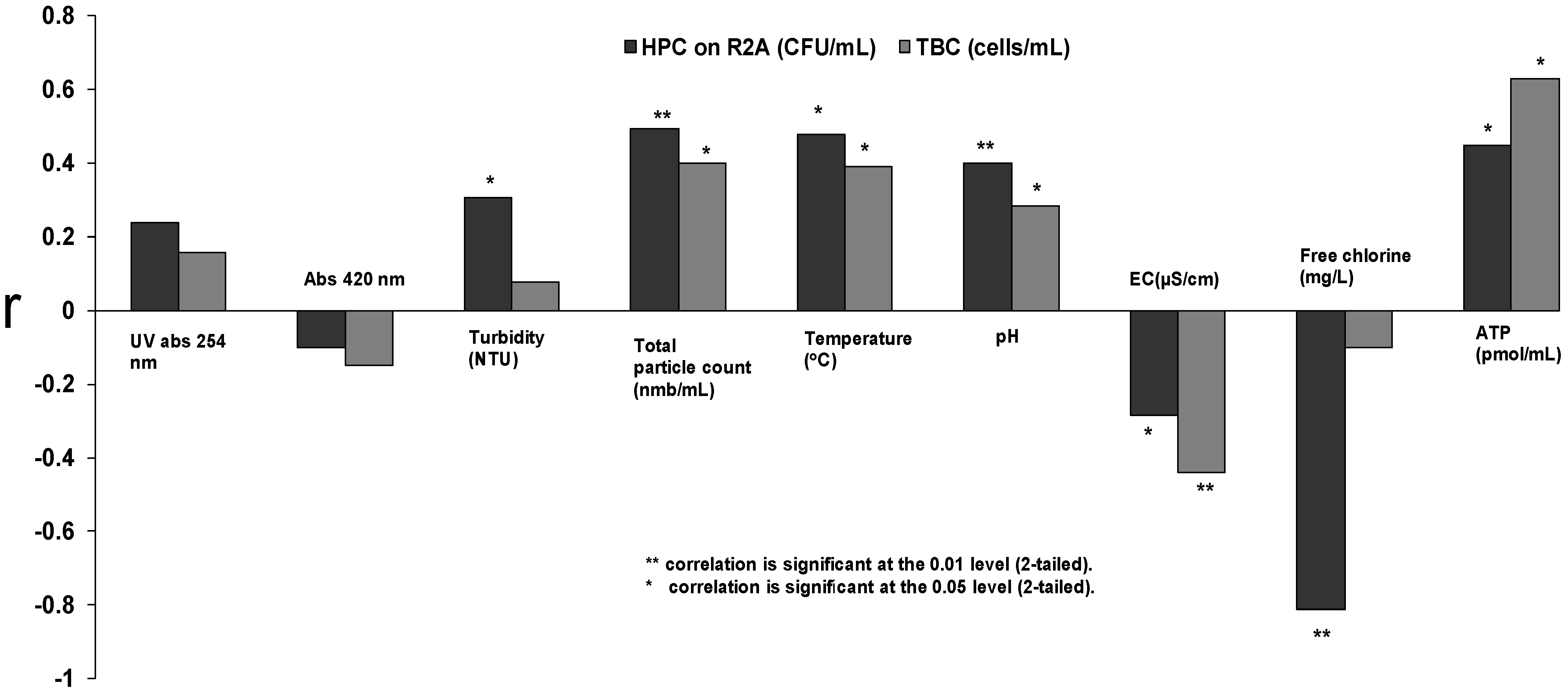
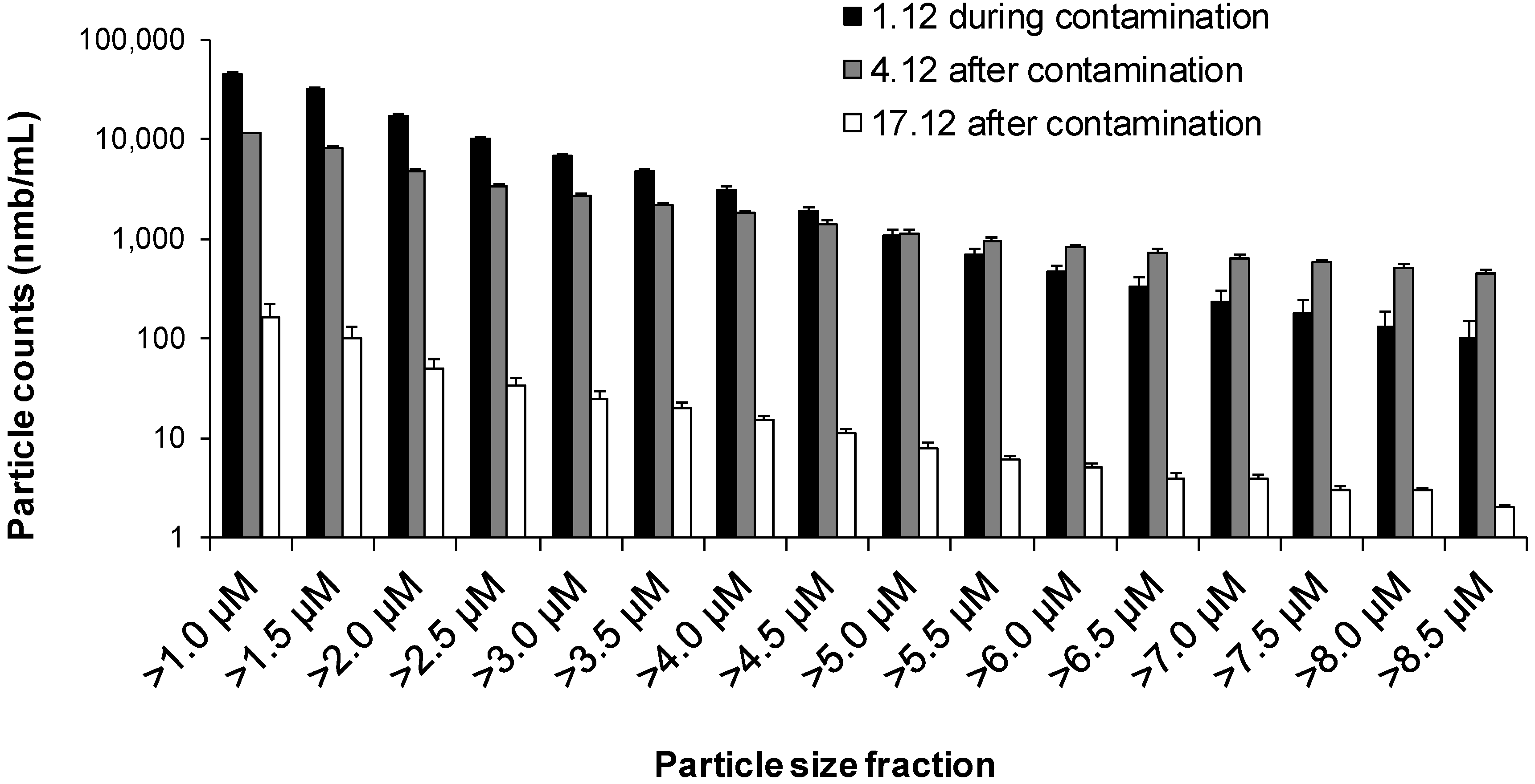
3.4. The Case of an Accidentally Contaminated Distribution Network
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- LeChevallier, M.W.; Welch, N.J.; Smith, D.B. Full-scale studies of factors related to coliform regrowth in drinking water. Appl. Environ. Microbiol. 1996, 62, 2201–2211. [Google Scholar]
- Gauthier, V.; Gerard, B.; Portal, J.; Block, J.; Gatel, D. Organic matter as loose deposits in a drinking water distribution system. Water Res. 1999, 33, 1014–1026. [Google Scholar] [CrossRef]
- Zacheus, O.M.; Lehtola, M.J.; Korhonen, L.K.; Martikainen, P.J. Soft deposits, the key site for microbial growth in drinking water distribution networks. Water Res. 2001, 35, 1757–1765. [Google Scholar]
- LeChevallier, M.W.; Babock, T.M.; Lee, R.G. Examination and characterization of distribution system biofilms. Appl. Environ. Microbiol. 1987, 53, 2714–2724. [Google Scholar]
- Miettinen, I.T.; Vartiainen, T.; Martikainen, P.J. Phosphorus and bacterial growth in drinking water. Appl. Environ. Microbiol. 1997, 63, 3242–3245. [Google Scholar]
- Geldreich, E.E. Coliform noncompliance nightmares in water supply distribution systems. In Water quality: A Realistic Perspective; College of Engineering, University of Michigan; Michigan Water Pollution Control Association; American Water Works Association, Michigan Department of Public Health: Lansing, Michigan, USA, 1988; pp. 55–74. [Google Scholar]
- Percival, S.T.; Walker, J.T.; Hunter, P.R. Biofilm Formation in Potable Water. In Microbial Aspects of Biofilms and Drinking Water; Vreeland, R.H., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 85–102. [Google Scholar]
- Roig, B.; Delpla, I.; Baures, E.; Jung, A.V.; Thomas, O. Analytical issues in monitoring drinking-water contamination related to short-term, heavy rainfall events. Trac-Trend. Anal. Chem. 2011, 30, 1243–1251. [Google Scholar] [CrossRef]
- Council Directive 98/83/EC on the Quality of Water Intended for Human Consumption. Available online: http://faolex.fao.org/cgi-bin/faolex.exe?rec_id=013657&database=FAOLEX&search_type=link&table=result&lang=eng&format_name=@ERALL (accessed on 21 October 2013).
- Reasoner, D.J.; Geldreich, E.E. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 1985, 49, 1–7. [Google Scholar]
- Water Quality, Enumeration of Culturable Micro-Organims. Colony Count by Inoculation in a Nutrient Agar Culture Medium; Finnish Standard Association (SFS): Helsinki, Finland, 1999.
- Reynolds, D.T.; Fricker, C.R. Application of laser scanning for the rapid and automated detection of bacteria in water samples. J. Appl. Microbiol. 1999, 86, 785–795. [Google Scholar] [CrossRef]
- Vital, M.; Dignum, M.; Magic-Knezev, A.; Ross, P.; Rietveld, L.; Hammes, F. Flow cytometry and Adenosine Tri-Phosphate analysis: Alternative possibilities to evaluate major bacteriological changes in drinking water treatment and distribution systems. Water Res. 2012, 46, 4665–4676. [Google Scholar] [CrossRef]
- Hobbie, J.E.; Daley, R.J.; Jasper, S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 1977, 33, 1225–1228. [Google Scholar]
- Kepner, R.L; Pratt, J.R. Use of fluorochromes for direct enumeration of total bacteria in environmental-samples—Past and present. Microbiol. Rev. 1994, 58, 603–615. [Google Scholar]
- Rinta-Kanto, J.M.; Lehtola, M.J.; Vartiainen, T.; Martikainen, P.J. Rapid enumeration of virus-like particles in drinking water samples using SYBR green I-staining. Water Res. 2004, 38, 2614–2618. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Pitkänen, T.; Miebach, L.; Miettinen, I.T. Survival of Campylobacter jejuni in potable water biofilms: A comparative study with different detection methods. Water Sci. Technol. 2006, 54, 57–61. [Google Scholar]
- van der Kooij, D.; Veenendaal, H.R.; Baars-Lorist, C.; van der Klift, D.W.; Drost, Y.C. Biofilm formation on surfaces of glass and Teflon exposed to treated water. Water Res. 1995, 29, 1655–1662. [Google Scholar]
- Delahaye, E.; Welte, B.; Levi, Y.; Leblon, G.; Montiel, A. An ATP-based method for monitoring the microbiological drinking water quality in a distribution network. Water Res. 2003, 37, 3689–3696. [Google Scholar] [CrossRef]
- Percival, S.T.; Walker, J.T.; Hunter, P.R. Methods of Sampling Biofilms in Potable Water. In Microbiological Aspects of Biofilms and Drinking Water; Vreeland, R.H., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 156–170. [Google Scholar]
- Berney, M.; Vital, M.; Hülshoff, I.; Weilenmann, H.; Egli, T.; Hammes, F. Rapid, cultivation-independent assessment of microbial viability in drinking water. Water Res. 2008, 42, 4010–4018. [Google Scholar] [CrossRef]
- Lopez-Roldan, R.; Tusell, P.; Courtois, S.; Luis, J.C. On-line bacteriological detection in water. Trac-Trend. Anal. Chem. 2013, 44, 46–57. [Google Scholar] [CrossRef]
- Pronk, M.; Goldscheider, N.; Zopfi, J. Dynamics and interaction of organic carbon, turbidity and bacteria in a Karst aquifer system. Hydrogeol. J. 2006, 14, 473–484. [Google Scholar] [CrossRef]
- Pronk, M.; Goldscheider, N.; Zopfi, J. Particle-size distribution as indicator for fecal bacteria contamination of drinking water from Karst springs. Environ. Sci. Technol. 2007, 41, 8400–8405. [Google Scholar] [CrossRef]
- van den Broeke, J.; Ross, P.S.; van der Helm, A.W.C.; Baars, E.T.; Rietveld, L.C. Use of on-line UV/Vis-spectrometry in the measurement of dissolved ozone and AOC concentrations in drinking water treatment. Water Sci. Technol. 2008, 57, 1169–1175. [Google Scholar] [CrossRef]
- Verberk, J.Q.J.C.; Hamilton, L.A.; O’Halloran, K.J.; van der Horst, W.; Vreeburg, J. Analysis of particle numbers, size and composition in drinking water transportation pipelines: Results of online measurements. Water Sci. Technol. 2006, 6, 35–43. [Google Scholar]
- Verberk, J.Q.J.C.; Vreeburg, J.H.G.; Rietveld, L.C.; van Dijk, J.C. Particulate fingerprinting of water quality in the distribution system. Water Sa 2009, 35, 192–199. [Google Scholar]
- Lehtola, M.J.; Pursiainen, A.; Hokajärvi, A-M.; Miettinen, I.T. Effects of the New Bank Filtrating Drinking Water Treatment Plant on Microbial Growth in Old Drinking Water Distribution System. In Proceeding of den 7 nordiske drickevanskonference, Copenhagen, Denmark, 7–9 June 2010; Danish Water and Wastewater Association: Skandenborg, Denmark, 2010; pp. 82–88. [Google Scholar]
- Miettinen, I.T.; Vartiainen, T.; Martikainen, P.J. Determination of assimilable organic carbon in humus-rich drinking waters. Water Res. 1999, 33, 2277–2282. [Google Scholar]
- Lehtola, M.J.; Miettinen, I.T.; Vartiainen, T.; Martikainen, P.J. A new sensitive bioassay for determination of microbially available phosphorus in water. Appl. Environ. Microbiol. 1999, 65, 2032–2034. [Google Scholar]
- Laine, J.; Huovinen, E.; Virtanen, M.J.; Snellman, M.; Lumio, J.; Ruutu, P.; Kujansuu, E.; Vuento, R.; Pitkänen, T.; Miettinen, I.; et al. An extensive gastroenteritis outbreak after drinking-water contamination by sewage effluent, Finland. Epidemiol. Infect. 2011, 139, 1105–1113. [Google Scholar] [CrossRef]
- Brenner, D.J.; Staley, J.T. The Alpha-, Beta-, Delta-, and Epsilonproteobacteria. In Bergey’s Manual of Systematic Bacteriology; Springer: New York, NY, USA, 2005. [Google Scholar]
- Block, J.C.; Haudidier, K.; Paquin, J.L.; Miazga, J.; Levi, Y. Biofilm accumulation in drinking water distribution systems. Biofouling 1993, 6, 333–343. [Google Scholar] [CrossRef]
- Flemming, H. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef]
- Sharp, E.L.; Parsons, S.A.; Jefferson, B. Seasonal variations in natural organic matter and its impact on coagulation in water treatment. Sci. Total Envir. 2006, 363, 183–194. [Google Scholar] [CrossRef]
- Chavez, A.; Jimenez, B.; Maya, C. Particle size distribution as a useful tool for microbial detection. Water Sci. Technol. 2004, 50, 179–186. [Google Scholar]
- Mccoy, W.F.; Olson, B.H. Relationship among turbidity, particle counts and bacteriological quality within water distribution lines. Water Res. 1986, 20, 1023–1029. [Google Scholar] [CrossRef]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Köster, O.; Egli, T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar]
- Liu, G.; van der Mark, E.J.; Verberk, J.Q.J.C.; van Dijk, J.C. Flow cytometry total cell counts: A field study assessing microbial water quality and growth in unchlorinated drinking water distribution systems. BioMed. Res. Int. 2013. [Google Scholar] [CrossRef]
- Francisque, A.; Rodriguez, M.J.; Miranda-Moreno, L.F.; Sadiq, R.; Proulx, F. Modeling of heterotrophic bacteria counts in a water distribution system. Water Res. 2009, 43, 1075–1087. [Google Scholar] [CrossRef]
- van der Kooij, D. Assimilable organic-carbon as an indicator of bacterial regrowth. J. Amer. Water Work. Assn. 1992, 84, 57–65. [Google Scholar]
- LeChevallier, M.W.; Lowry, C.D.; Lee, R.G. Disinfecting biofilms in a model distribution-system. J. Amer. Water Work. Assn. 1990, 82, 87–99. [Google Scholar]
- Lehtola, M.J.; Miettinen, K.T.; Keinänen, M.M.; Kekki, T.K.; Laine, O.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. Microbiology, chemistry and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res. 2004, 38, 3769–3779. [Google Scholar]
- Helbling, D.E.; VanBriesen, J.M. Continuous monitoring of residual chlorine concentrations in response to controlled microbial intrusions in a laboratory-scale distribution system. Water Res. 2008, 42, 3162–3172. [Google Scholar] [CrossRef]
- Carter, J.; Rice, E.; Buchberger, S.; Lee, Y. Relationships between levels of heterotrophic bacteria and water quality parameters in a drinking water distribution system. Water Res. 2000, 34, 1495–1502. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Disinfection. In Water Treatment: Principles and Design; Wiley: Somerset, NJ, USA, 2005; pp. 1035–1162. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ikonen, J.; Pitkänen, T.; Miettinen, I.T. Suitability of Optical, Physical and Chemical Measurements for Detection of Changes in Bacterial Drinking Water Quality. Int. J. Environ. Res. Public Health 2013, 10, 5349-5363. https://doi.org/10.3390/ijerph10115349
Ikonen J, Pitkänen T, Miettinen IT. Suitability of Optical, Physical and Chemical Measurements for Detection of Changes in Bacterial Drinking Water Quality. International Journal of Environmental Research and Public Health. 2013; 10(11):5349-5363. https://doi.org/10.3390/ijerph10115349
Chicago/Turabian StyleIkonen, Jenni, Tarja Pitkänen, and Ilkka T. Miettinen. 2013. "Suitability of Optical, Physical and Chemical Measurements for Detection of Changes in Bacterial Drinking Water Quality" International Journal of Environmental Research and Public Health 10, no. 11: 5349-5363. https://doi.org/10.3390/ijerph10115349
APA StyleIkonen, J., Pitkänen, T., & Miettinen, I. T. (2013). Suitability of Optical, Physical and Chemical Measurements for Detection of Changes in Bacterial Drinking Water Quality. International Journal of Environmental Research and Public Health, 10(11), 5349-5363. https://doi.org/10.3390/ijerph10115349





