Effect of the Environmental Pollutant Hexachlorobenzene (HCB) on the Neuronal Differentiation of Mouse Embryonic Stem Cells †
Abstract
:1. Introduction
2. Experimental Section
2.1. ES Cell Culture
2.2. GABAergic and Glutamatergic Neuronal Differentiation
2.3. HCB Treatment and Cell Viability Determination
2.4. Immunofluorescence Analyses
2.5. RT-PCR Analyses
2.6. ROS Assay
2.7. NAC Treatment
2.8. Neurite Lenght Measurements
2.9. Electrophysiology Recordings
3. Results
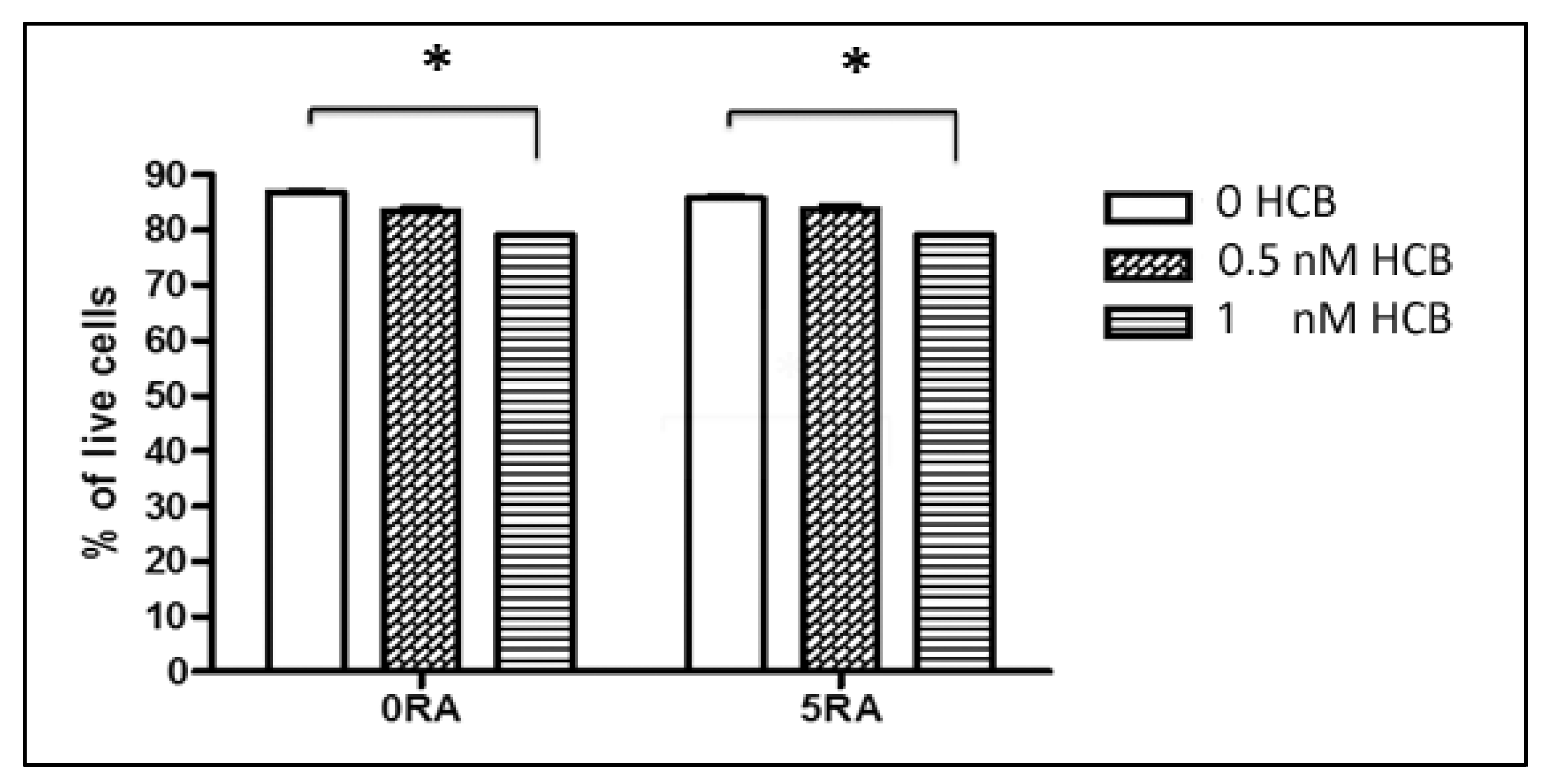
3.1. Dose-Response Effect of HCB on Cell Viability
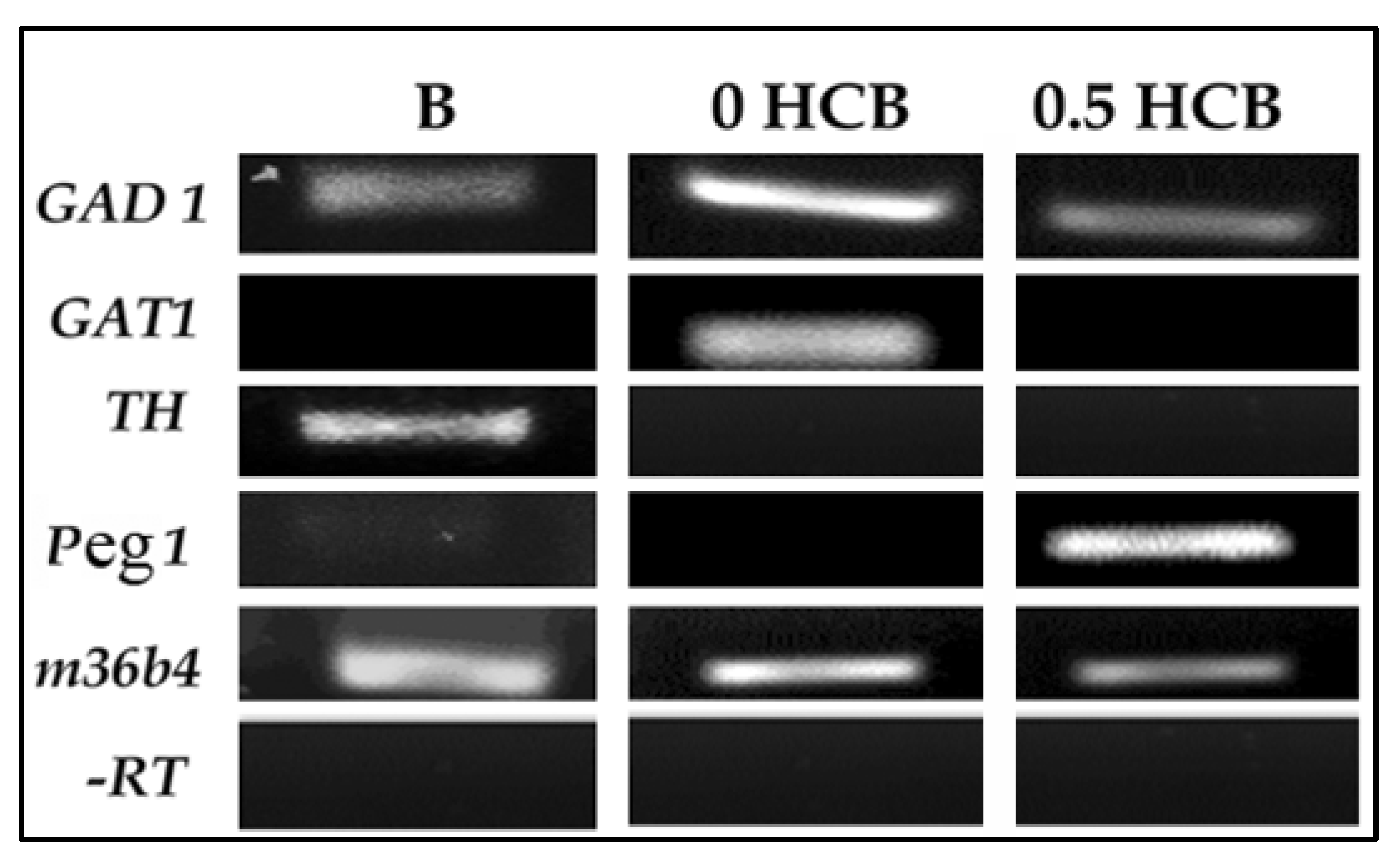
3.2. Expression of Lineage Markers in Encapsulated ES Cells by RT-PCR
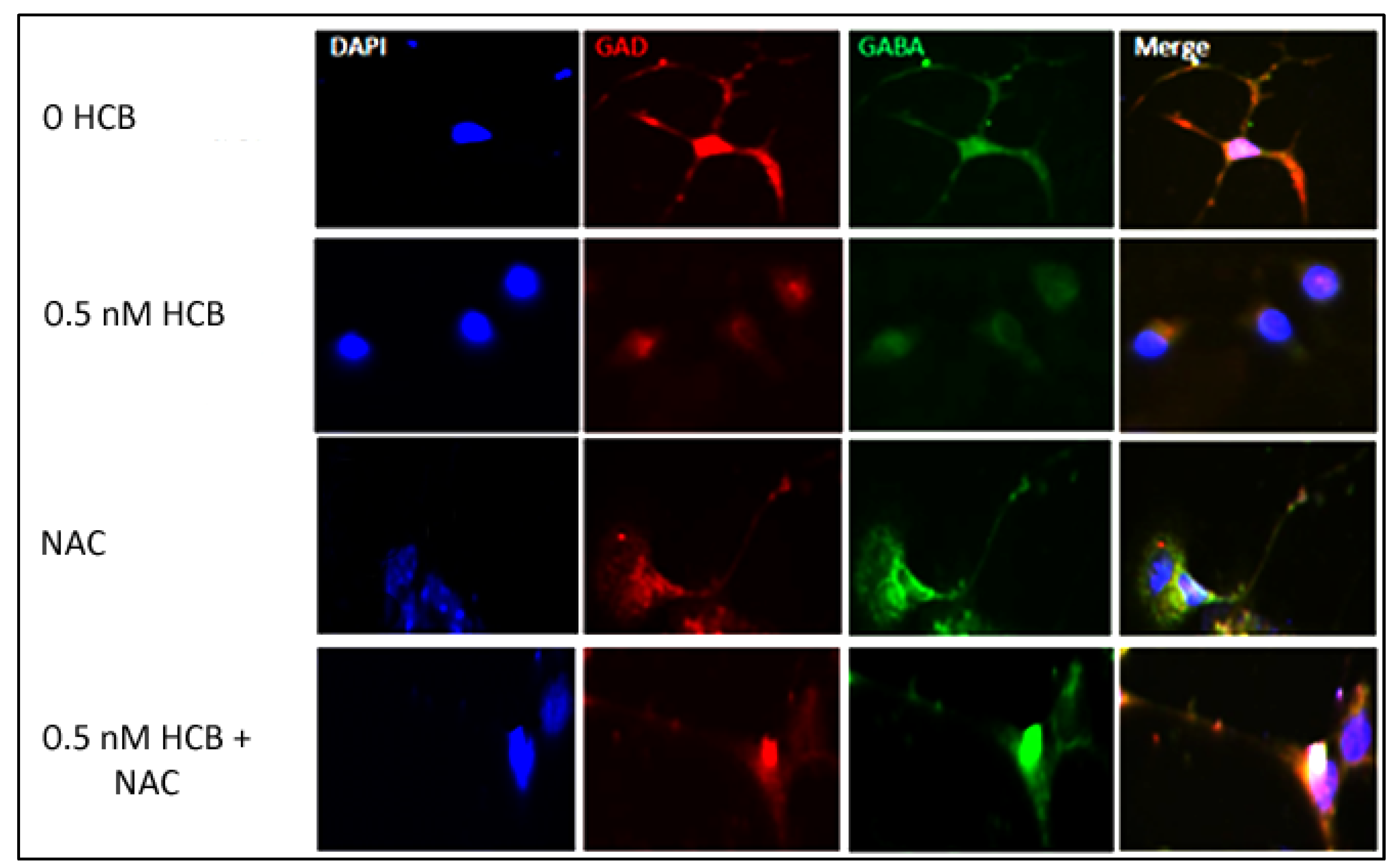
3.3. Low Dose HCB Affects GABAergic Neuronal Maturation

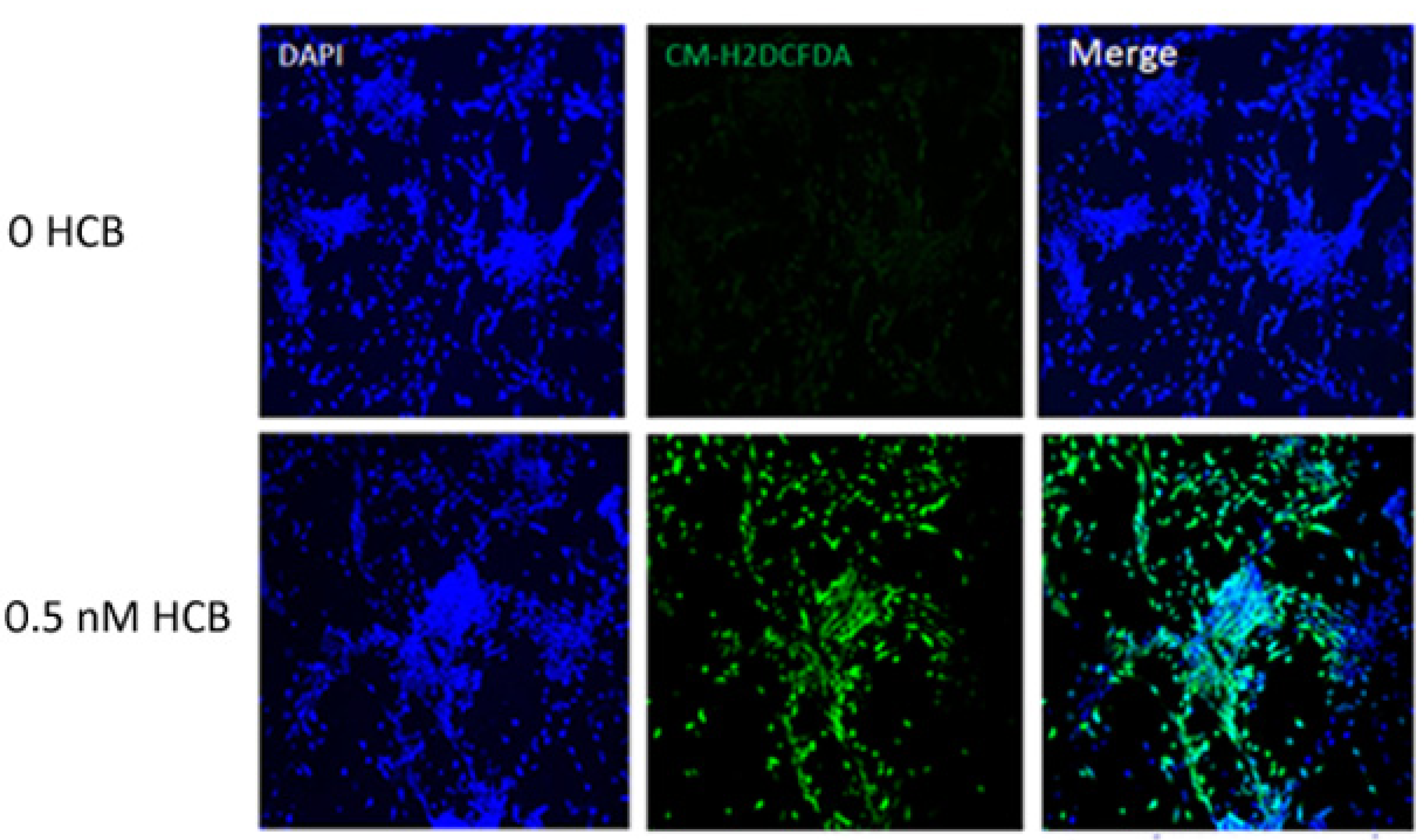
3.4. HCB Induces ROS Production in GABAergic Cells
3.5. Inhibition of ROS Production Restores Neurite Outgrowth in HCB-Treated Neurons
3.6. Electrophysiology Analyses

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Williams, R.L.; Hilton, D.J.; Pease, S.; Willson, T.A.; Stewart, C.L.; Gearing, D.P.; Wagner, E.F.; Metcalf, D.; Nicola, N.A.; Gough, N.M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988, 336, 684–687. [Google Scholar]
- Lindvall, O. Stem cells for cell therapy in Parkinson’s disease. Pharmacol. Res. 2003, 47, 279–287. [Google Scholar] [CrossRef]
- Goldman, S.A.; Windrem, M.S. Cell replacement therapy in neurological disease. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006, 361, 1463–1475. [Google Scholar] [CrossRef]
- Toxicological Profile for Hexachlorobenzene. Available online: www.atsdr.cdc.gov/toxprofiles/tp.asp?id = 627&tid = 115 (accessed on 30 December 2012).
- Bradman, A.S.; Schwartz, J.M.; Fenster, L.; Barr, D.B.; Holland, N.T.; Eskenazi, B. Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 388–399. [Google Scholar]
- Reed, L.; Buchner, V.; Tchounwou, P.B. Environmental toxicology and health effects associated with hexachlorobenzene exposure. Rev. Environ. Health 2007, 22, 213–243. [Google Scholar]
- Johnson, W.W.; Finley, M.T. Handbook of Acute Toxicity of Chemicals to Fish and Aquatic Invertebrates; U. S. Department of the Interior, Fish and Wildlife Service: Columbia, MO, USA, 1980. [Google Scholar]
- Hexachlorobenzene in Drinking-Water. Available online: www.who.int/water_sanitation_health/dwq/chemicals/hexachlorobenzene.pdf (accessed on 30 December 2012).
- Liu, J.; Schelar, E. Pesticide exposure and child neurodevelopment: Summary and implications. Workplace Health Saf. 2012, 60, 235–242. [Google Scholar]
- Lopez-Espinosa, M.J.; Vizcaino, E.; Murcia, M.; Fuentes, V.; Garcia, A.M.; Rebagliato, M.; Grimalt, J.O.; Ballester, F. Prenatal exposure to organochlorine compounds and neonatal thyroid stimulating hormone levels. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 579–588. [Google Scholar]
- van Birgelen, A.P. Hexachlorobenzene as a possible major contributor to the dioxin activity of human milk. Environ. Health Perspect. 1998, 106, 683–688. [Google Scholar] [CrossRef]
- Plante, I.; Charbonneau, M.; Cyr, D.G. Decreased gap junctional intercellular communication in hexachlorobenzene-induced gender-specific hepatic tumor formation in the rat. Carcinogenesis 2002, 23, 1243–1249. [Google Scholar] [CrossRef]
- Pontillo, C.A.; Garcia, M.A.; Pena, D.; Cocca, C.; Chiappini, F.; Alvarez, L.; Kleiman de Pisarev, D.; Randi, A.S. Activation of c-Src/HER1/STAT5b and HER1/ERK1/2 signaling pathways and cell migration by hexachlorobenzene in MDA-MB-231 human breast cancer cell line. Toxicol. Sci. 2011, 120, 284–296. [Google Scholar]
- Salmon, M.L.; Madanagopal, S.G.; Blando, R.; Berner, J.; Williams, K. Effects of hexachlorobenzene on embryonic mammalian cells. Toxicol. In Vitro 2002, 16, 539–548. [Google Scholar]
- Martinez-Ceballos, E.; Chambon, P.; Gudas, L.J. Differences in gene expression between wild type and Hoxa1 knockout embryonic stem cells after retinoic acid treatment or leukemia inhibitory factor (LIF) removal. J. Biol. Chem. 2005, 280, 16484–16498. [Google Scholar] [CrossRef]
- Addae, C.; Yi, X.; Gernapudi, R.; Cheng, H.; Musto, A.; Martinez-Ceballos, E. All-trans-retinoid acid induces the differentiation of encapsulated mouse embryonic stem cells into GABAergic neurons. Differentiation 2012, 83, 233–241. [Google Scholar] [CrossRef]
- Martinez-Ceballos, E.; Gudas, L.J. Hoxa1 is required for the retinoic acid-induced differentiation of embryonic stem cells into neurons. J. Neurosci. Res. 2008, 86, 2809–2819. [Google Scholar] [CrossRef]
- Loaiza-Perez, A.I.; Seisdedos, M.T.; Kleiman de Pisarev, D.L.; Sancovich, H.A.; Randi, A.S.; Ferramola de Sancovich, A.M.; Santisteban, P. Hexachlorobenzene, a dioxin-type compound, increases malic enzyme gene transcription through a mechanism involving the thyroid hormone response element. Endocrinology 1999, 140, 4142–4151. [Google Scholar] [CrossRef] [Green Version]
- Shadnia, S.; Azizi, E.; Hosseini, R.; Khoei, S.; Fouladdel, S.; Pajoumand, A.; Jalali, N.; Abdollahi, M. Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum. Exp. Toxicol. 2005, 24, 439–445. [Google Scholar]
- Khan, S.; Ahmad, T.; Parekh, C.V.; Trivedi, P.P.; Kushwaha, S.; Jena, G. Investigation on sodium valproate induced germ cell damage, oxidative stress and genotoxicity in male Swiss mice. Reprod. Toxicol. 2011, 32, 385–394. [Google Scholar] [CrossRef]
- Nisenbaum, E.S.; Wilson, C.J. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. 1995, 15, 4449–4463. [Google Scholar]
- Falk, T.; Zhang, S.; Erbe, E.L.; Sherman, S.J. Neurochemical and electrophysiological characteristics of rat striatal neurons in primary culture. J. Comp. Neurol. 2006, 494, 275–289. [Google Scholar]
- Chen, L.F.; Yin, Z.Q.; Chen, S.; Chen, Z.S. Differentiation and production of action potentials by embryonic rat retina stem cells in vitro. Invest. Ophthalmol. Vis. Sci. 2008, 49, 5144–5150. [Google Scholar]
- Cripps, D.J.; Peters, H.A.; Gocmen, A.; Dogramici, I. Porphyria turcica due to hexachlorobenzene: A 20 to 30 year follow-up study on 204 patients. Br. J. Dermatol. 1984, 111, 413–422. [Google Scholar] [CrossRef]
- Gocmen, A.; Peters, H.A.; Cripps, D.J.; Morris, C.R.; Dogramaci, I. Porphyria turcica: Hexachlorobenzene-induced porphyria. IARC Sci. Publ. 1986, 567–573. [Google Scholar]
- Lilienthal, H.; Benthe, C.; Heinzow, B.; Winneke, G. Impairment of schedule-controlled behavior by pre- and postnatal exposure to hexachlorobenzene in rats. Arch. Toxicol. 1996, 70, 174–181. [Google Scholar] [CrossRef]
- Valkusz, Z.; Nagyeri, G.; Radacs, M.; Ocsko, T.; Hausinger, P.; Laszlo, M.; Laszlo, F.A.; Juhasz, A.; Julesz, J.; Palfoldi, R.; Galfi, M. Further analysis of behavioral and endocrine consequences of chronic exposure of male Wistar rats to subtoxic doses of endocrine disruptor chlorobenzenes. Physiol. Behav. 2011, 103, 421–430. [Google Scholar] [CrossRef]
- Randi, A.S.; Cocca, C.; Carbone, V.; Nunez, M.; Croci, M.; Gutierrez, A.; Bergoc, R.; Kleiman de Pisarev, D.L. Hexachlorobenzene is a tumor co-carcinogen and induces alterations in insulin-growth factors signaling pathway in the rat mammary gland. Toxicol. Sci. 2006, 89, 83–92. [Google Scholar]
- Kawaguchi, J.; Mee, P.J.; Smith, A.G. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone 2005, 36, 758–769. [Google Scholar] [CrossRef]
- Randi, A.S.; Sanchez, M.S.; Alvarez, L.; Cardozo, J.; Pontillo, C.; Kleiman de Pisarev, D.L. Hexachlorobenzene triggers AhR translocation to the nucleus, c-Src activation and EGFR transactivation in rat liver. Toxicol. Lett. 2008, 177, 116–122. [Google Scholar] [CrossRef]
- Dey, N.; De, P.K.; Wang, M.; Zhang, H.; Dobrota, E.A.; Robertson, K.A.; Durden, D.L. CSK controls retinoic acid receptor (RAR) signaling: A RAR-c-SRC signaling axis is required for neuritogenic differentiation. Mol. Cell Biol. 2007, 27, 4179–4197. [Google Scholar] [CrossRef]
- Balamurugan, K.; Rajaram, R.; Ramasami, T.; Narayanan, S. Chromium(III)-induced apoptosis of lymphocytes: Death decision by ROS and Src-family tyrosine kinases. Free Radic. Biol. Med. 2002, 33, 1622–1640. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Evers, B.M.; Chung, D.H. Signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis. Pediatr. Res. 2005, 58, 1192–1197. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Addae, C.; Cheng, H.; Martinez-Ceballos, E. Effect of the Environmental Pollutant Hexachlorobenzene (HCB) on the Neuronal Differentiation of Mouse Embryonic Stem Cells. Int. J. Environ. Res. Public Health 2013, 10, 5244-5256. https://doi.org/10.3390/ijerph10105244
Addae C, Cheng H, Martinez-Ceballos E. Effect of the Environmental Pollutant Hexachlorobenzene (HCB) on the Neuronal Differentiation of Mouse Embryonic Stem Cells. International Journal of Environmental Research and Public Health. 2013; 10(10):5244-5256. https://doi.org/10.3390/ijerph10105244
Chicago/Turabian StyleAddae, Cynthia, Henrique Cheng, and Eduardo Martinez-Ceballos. 2013. "Effect of the Environmental Pollutant Hexachlorobenzene (HCB) on the Neuronal Differentiation of Mouse Embryonic Stem Cells" International Journal of Environmental Research and Public Health 10, no. 10: 5244-5256. https://doi.org/10.3390/ijerph10105244



