Mitogenic and Cytotoxic Effects of Pentachlorophenol to AML 12 Mouse Hepatocytes
Abstract
:Introduction
Materials and Methods
Chemicals and Reagents
Preparation and Culture of AML 12 Mouse Hepatocytes
Cell Viability Experiments for AML 12 Mouse Hepatocytes
Statistical Analysis
Results
Cytotoxicity Assay
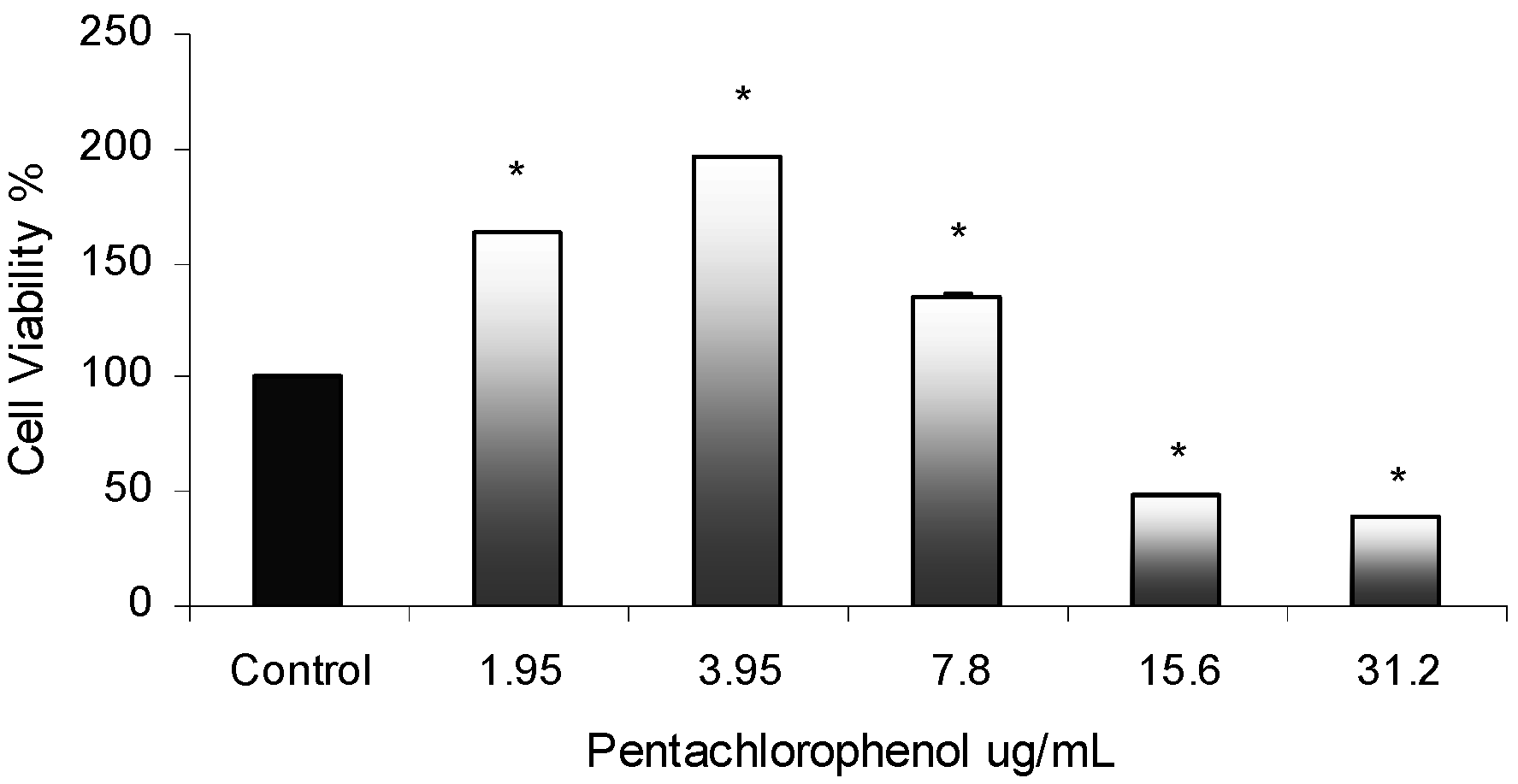
Mitogenic Activity in PCP-Treated AML 12 Mouse Hepatocytes

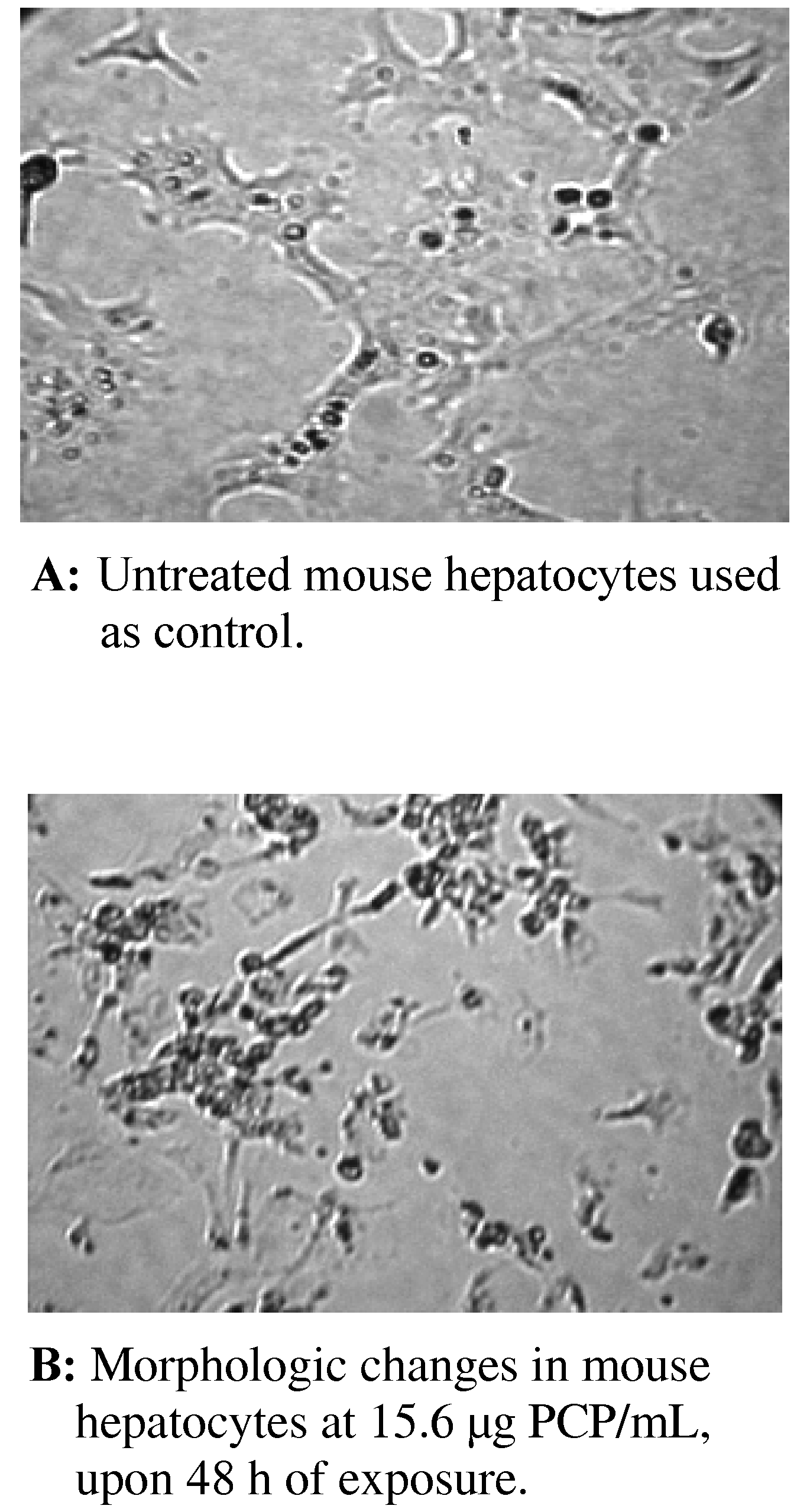
Morphologic Changes in PCP-Treated AML 12 Mouse Hepatocytes
Discussion
PCP-Induced Cytotoxic Effects in AML 12 Mouse Hepatocytes
Mitogenic Activity of PCP in AML 12 Mouse Hepatocytes
Morphologic Changes in AML 12 Mouse Hepatocytes
Conclusions
Acknowledgments
References
- Dorsey, W. C.; Tchounwou, P. B. CYP1A1, Hsp70, p53, and c-fos expression in human liver carcinoma cells (HepG2) exposed to pentachlorophenol. Biomedical Research Instrumentation 2003, 437, 389–396. [Google Scholar]
- Dorsey, W. C.; Tchounwou, P. B. Pentachlorophenol - induced cytotoxic, mitogenic, and endocrine-disrupting activities in channel catfish, Ictalurus punctatus. International J. Environ. Research and Public Health 2004, 2, 74–83. [Google Scholar]
- Fisher, B. Pentachlorophenol: toxicology and environmental fate. J. Pesticide Reform. 1991, 11(1), 1–5. [Google Scholar]
- Kavlock, R.; Ankley, G.; Francis, E.; Grag, E.; McMaster, S.; Reese, D.; Sayles, G.; Sergeant, A.; Vallero, D. Research plan for endocrine disruptors: a report of the U.S. EPA. EPA600R98087, 1998. [Google Scholar]
- Patino, R.; Maule, A. G. Estrogen receptors in leukocytes from immature channel catfish. Dev. Comp. Immunol. 1997, 2, 123–24. [Google Scholar] [CrossRef]
- Colborn, T.; vom Saal, F. S.; Soto, A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspectives 1993, 101(5), 256–257. [Google Scholar]
- Meacher, M. Speech to SERA Conference; 25th January 1997.
- De Solla, S. R.; Bishop, C. A.; Van Der Kraak, G.; Brooks, R. J. Impact of organochlorine contamination on levels of sex hormones and external morphology of common snapping turtles (Chelydtra serpentina serpentina) in Ontario, Canada. Environ. Health Perspectives. 1998, 106, 253–260. [Google Scholar] [CrossRef]
- U. S. Environmental Protection Agency. Treatment technology performance and cost data for remediation of wood preserving sites. EPA625R97007, Office of Research and Development: Washington, D.C., 1997. [Google Scholar]
- National Toxicology Program: NTP toxicology and carcinogenesis studies of penta- chlorophenol (CAS No. 87-86-5) in F344/N Rats (Feed Studies). Natl Toxicol Program Tech. Rep. Ser. 1999, 483, 1–182.
- U. S. Environmental Protection Agency. Pentachlorophenol (non wood uses) special review position document 2/3; Office of Pesticides and Toxic Substances: Washington, D.C., 1984; pp. 15–16. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. ToxFAQs(tm) for Pentachloro-phenol CAS No. 87-86-5. URL: www.astr.cdc.gov/tfacts51.html 2001.
- Daniel, V.; Wolfgang, H.; Klausdieter, B.; Opelz, G. Impaired in-vitro lymphocyte responses in patients with elevated pentachlorophenol (PCP) blood levels. Archives of Environmental Health. 1995, 50, 287–292. [Google Scholar] [CrossRef]
- Rugman, F. P.; Cosstick, R. Aplastic anemia associated with organochlorine pesticide: case reports and review of evidence. J. Clinical Pathology 2000, 43, 98–101. [Google Scholar] [CrossRef]
- Winsholz, M. The Merck Index. 10th ed. Merck and Co.: Rahway, NJ, 1983; 1021. [Google Scholar]
- Wagner, S. L. Clinical Toxicology of Agricultural Chemicals. Oregon State University Environmental Health Sciences Center: Corvallis, OR, 1981. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). In Toxicological Profile for Pentachlorophenol (Draft); U.S. Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA; p. 1992.
- Spalding, J. W.; French, J. E.; Stasiewicz, S.; Furedi-Machacek, M.; Conner, F.; Tice, R. R.; Tennant, R. W. Responses of transgenic mouse lines p53 (+/-) and Tg.AC to agents tested in conventional carcinogenicity bioassays. Toxicol. Sci. 2000, 53, 213–223. [Google Scholar] [CrossRef]
- Chhabra, R. S.; Maronpot, R. M.; Bucher, J. R.; Haseman, J. K.; Toft, J. D.; Hejtmancik, M. R. Toxicology and carcinogenesis studies of pentachlorophenol in rats. Toxicol. Sci. 1999, 48, 14–20. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Pentachlorophenol; Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development: Cincinnati, OH, 1993. [Google Scholar]
- Pope, A.; Rall, D. Environmental medicine: integrating a missing element into medical education. Committee on Curriculum Development in Environmental Medicine. Institute of Medicine. National Academy Press 1995, 542–555. [Google Scholar]
- Colosio, C.; Maroni, M.; Barcellini, W.; Meroni, P.; Alcini, D.; Colombi, A. D.; Foa, V. Toxicological and immune findings in workers exposed to penta-chlorophenol (PCP). Archives of Environ. Health 1993, 48(2), 81–88. [Google Scholar]
- Wood, S.; Rom, V. N.; White, G. L.; Logan, D. C. Pentachlorophenol poisoning. J. Occupational Medicine 1983, 25, 527–30. [Google Scholar]
- Dorsey, W. C.; Tchounwou, P. B.; Ishaque, A. B.; Shen, E. Transcriptional activation of stress genes and cytotoxicity in human liver carcinoma (HepG2) exposed to pentachlorophenol. International J. Molecular Sci. 2002, 3, 989–1004. [Google Scholar]
- Burow, M. E.; Yang, T.; Collins-Burow, B. M.; Krajewski, S.; Reed, J. C.; McLachlan, J. A.; Beckman, B. S. Effects of environmental estrogens on tumor necrosis factor α-mediated apoptosis in MC7-cells. Carcinogenesis 1999, 20(11), 2057–2061. [Google Scholar]
- Marino, M.; Acconcia, F.; Bresciana, R.; Weiz, A.; Trentalance, A. Distinct non- genomic signal transduction pathways controlled by 17ß-estradiol regulate DNA synthesis and cyclin D1 gene transcription in HepG2 cells. Molecular Biology of the Cell 2002, 13, 3720–3729. [Google Scholar] [CrossRef]
- Sasaki, Y.; Sugamura, K. Involvement of Hgs/Hrs in signaling for cytokine-c-fos induction through interaction with TAK1 and Pak1. J. Bio. Chem. 2001, 276, 2999433–29952. [Google Scholar]
- Derijard, B.; Hibi, M.; Wu, I. -H.; Barrett, T.; Su, B.; Deng, T.; Karin, M.; Davis, R. J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 1994, 76, 1025–1037. [Google Scholar] [CrossRef]
- Firpo, A. Cell injury. Cornell University Medical College Education Center. URL: http://edcenter.med.cornell.edu/CUMC_PathNotes/Cell.Injury/Cell_Injury.html.
© 2004 MDPI. All rights reserved.
Share and Cite
Dorsey, W.C.; Tchounwou, P.B.; Sutton, D. Mitogenic and Cytotoxic Effects of Pentachlorophenol to AML 12 Mouse Hepatocytes. Int. J. Environ. Res. Public Health 2004, 1, 100-105. https://doi.org/10.3390/ijerph2004020100
Dorsey WC, Tchounwou PB, Sutton D. Mitogenic and Cytotoxic Effects of Pentachlorophenol to AML 12 Mouse Hepatocytes. International Journal of Environmental Research and Public Health. 2004; 1(2):100-105. https://doi.org/10.3390/ijerph2004020100
Chicago/Turabian StyleDorsey, Waneene C., Paul B. Tchounwou, and Dwayne Sutton. 2004. "Mitogenic and Cytotoxic Effects of Pentachlorophenol to AML 12 Mouse Hepatocytes" International Journal of Environmental Research and Public Health 1, no. 2: 100-105. https://doi.org/10.3390/ijerph2004020100




