Halogenated Compounds from Marine Algae
Abstract
:1. Introduction
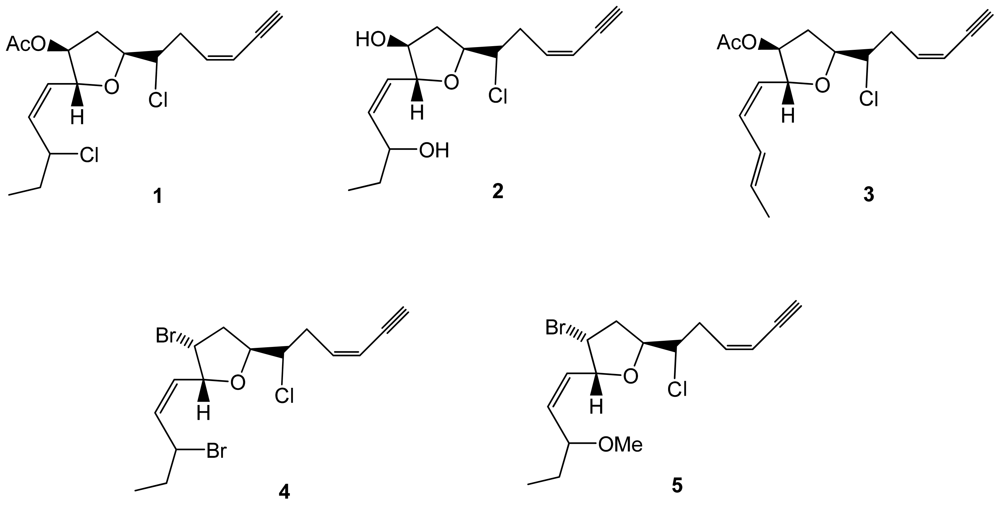
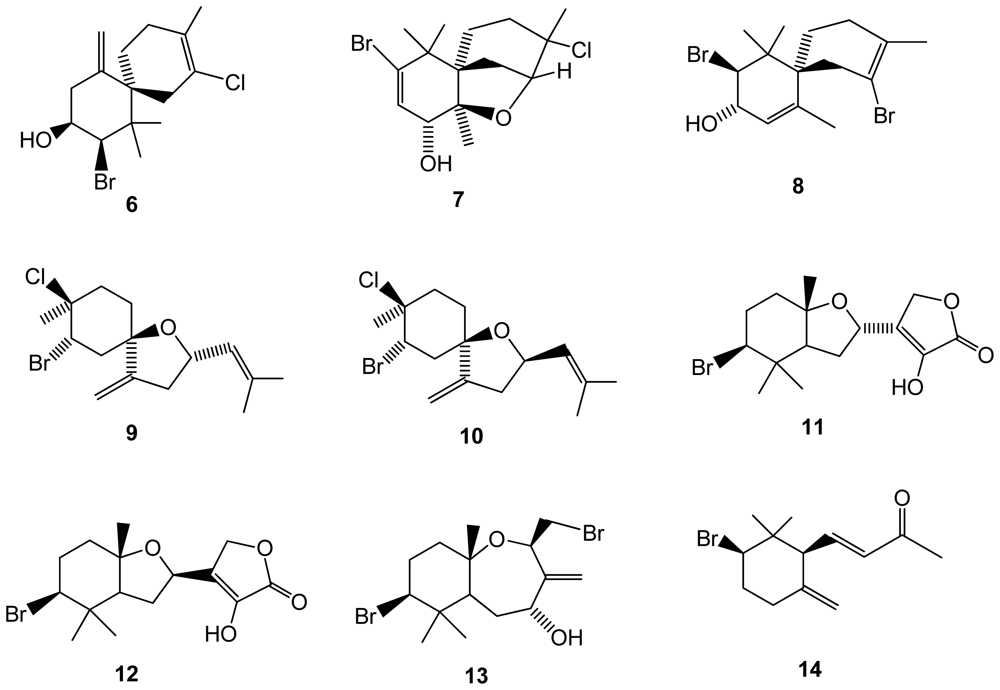
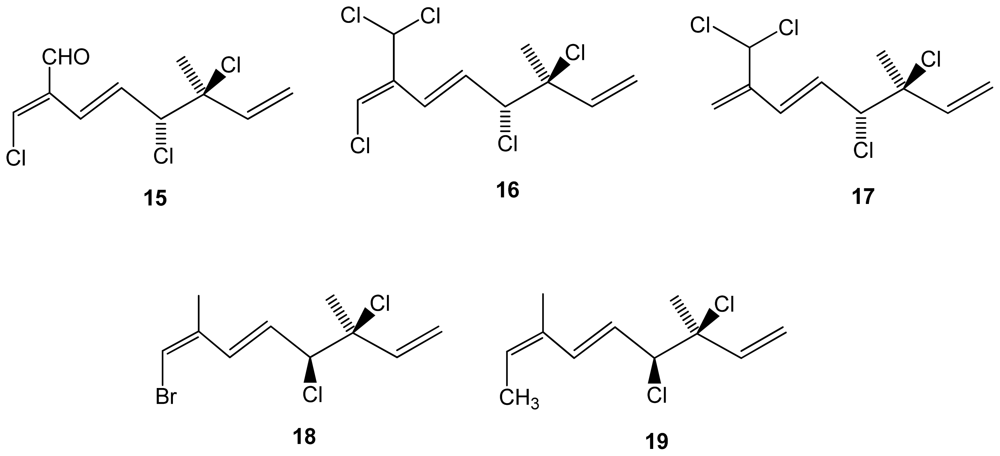
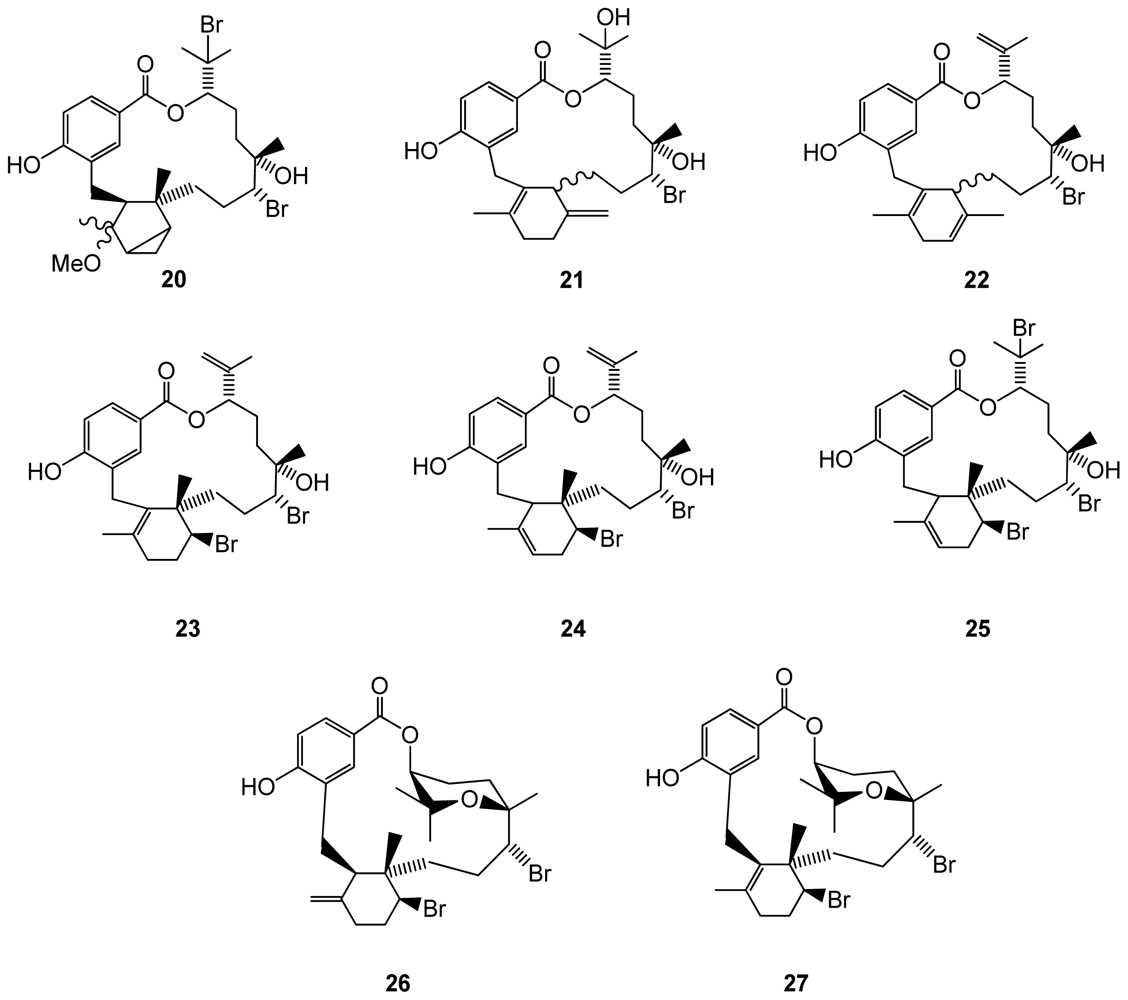
2. Macroalgae
3. Cyanobacteria
4. Limitations and Possible Solutions to Detection of Bioactivity
5. Ecological Role
6. Present Trends
Acknowledgements
References
- Cimino, G; Ghiselin, MT. McClintock, JB, Baker, BJ, Eds.; Marine natural products chemistry as an evolutionary narrative. In Marine Chemical Ecology; CRC Press: Boca Raton, FL, USA, 2001; pp. 115–154. [Google Scholar]
- Cannell, RJP. Cannell, RPJ, Ed.; How to approach the isolation of a natural product. In Natural Products Isolation, Methods in Biotechnology; Humana Press: Totowa, NJ, USA, 1998; Volume 4, pp. 1–51. [Google Scholar]
- Ianora, A; Boersma, M; Casotti, R; Fontana, A; Harder, J; Hoffmann, F; Pavia, H; Potin, P; Poulet, SA; Toth, G. New trends in marine chemical ecology. Estuaries Coasts 2006, 29, 531–551. [Google Scholar]
- Butler, A; Sandy, M. Mechanistic considerations of halogenating enzymes. Nature 2009, 460, 848–854. [Google Scholar]
- Neumann, CS; Fujimori, DG; Walsh, CT. Halogenation strategies in natural product biosynthesis. Chem. Biol 2008, 15, 99–109. [Google Scholar]
- Küpper, FC; Schweigert, N; Ar Gall, E; Legendre, J-M; Vilter, H; Kloareg, B. Iodine uptake in Laminariales involves extracellular, haloperoxidase-mediated oxidation of iodide. Planta 1998, 207, 163–167. [Google Scholar]
- Harper, MK; Bugni, TS; Copp, BR; James, RD; Lindsay, BS; Richardson, AD; Schnabel, PC; Tasdemir, D; Van Wagoner, RM; Verbitzki, SM; Ireland, CM. McClintock, JB, Baker, BJ, Eds.; Introduction to the chemical ecology of marine natural products. In Marine Chemical Ecology; CRC: Boca Raton, FL, USA, 2001; pp. 3–71. [Google Scholar]
- Renner, MK; Jensen, PR; Fenical, W. Neomangicols: structures and absolute stereochemistries of unprecedented halogenated sesterterpenes from a marine fungus of the genus Fusarium. J. Org. Chem 1998, 63, 8346–8354. [Google Scholar]
- Blunt, JW; Copp, BR; Hu, WP; Munro, MHG; Northcote, PT; Prinsep, MR. Marine natural products. Nat. Prod. Rep 2009, 26, 170–244. [Google Scholar]
- Fenical, W; Norris, JN. Chemotaxonomy in marine algae: chemical separation of some Laurencia species (Rhodophyta) from the Gulf of California). J. Phycol 1975, 11, 104–108. [Google Scholar]
- Hay, ME; Fenical, W; Gustafson, K. Chemical defense against diverse coral-reef herbivores. Ecology 1987, 68, 1581–1591. [Google Scholar]
- Vairappan, CS. Potent antibacterial activity of halogenated metabolites from Malaysian red algae, Laurencia majuscula (Rhodomelaceae, Ceramiales). Biomol. Eng 2003, 20, 255–259. [Google Scholar]
- Vairappan, CS; Ang, MY; Ong, CY; Phang, SM. Biologically active polybrominated indoles in the red alga Laurencia similis from the coastal waters of Sabah (Rhodomelaceae, Ceramiales). Malaysian J. Sci 2004, 23, 119–126. [Google Scholar]
- Paul, NA; de Nys, R; Steinberg, PD. Seaweed–herbivore interactions at a small scale: direct tests of feeding deterrence by filamentous algae. Mar. Ecol. Prog. Ser 2006, 323, 1–9. [Google Scholar]
- Harlin, MM; Rice, EL. Allelochemistry in marine macroalgae. Crit. Rev. Plant Sci 1987, 3, 237–249. [Google Scholar]
- Bolser, RC; Hay, ME. Are tropical plants better defended? Palatability and defenses of temperate vs. tropical seaweeds. Ecology 1996, 77, 2269–2286. [Google Scholar]
- Hay, ME. Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Annu. Rev. Mar. Sci 2009, 1, 193–212. [Google Scholar]
- Blunt, JW; Copp, BR; Hu, W-P; Munro, MHG; Northcote, PT; Prinsep, MR. Marine natural products. Nat. Prod. Rep 2007, 24, 31–86. [Google Scholar]
- Hill, RA. Marine natural products. Annu. Rep. Prog. Chem., Sect. B: Org. Chem 2007, 103, 125–139. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat. Prod. Rep 2001, 18, 1–49. [Google Scholar]
- Wright, AD; Goclik, E; König, GM. Three new sesquiterpenes from the red alga Laurencia perforate. J. Nat. Prod 2003, 66, 435–437. [Google Scholar]
- Erickson, KL. Scheuer, PJ, Ed.; Constituents of Laurencia. In Marine Natural Products: Chemical and Biological Perspectives; Academic Press: New York, NY, USA, 1983; Volume 5, pp. 131–257. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat. Prod. Rep 1995, 12, 223–269. [Google Scholar]
- König, GM; Wright, AD. Laurencia rigida: Chemical investigations of its antifouling dichloromethane extract. J. Nat. Prod 1997, 60, 967–970. [Google Scholar]
- Kurata, K; Taniguchi, K; Agatsuma, Y; Suzuki, M. Diterpenoid feeding-deterrents from Laurencia saitoi. Phytochemistry 1998, 47, 363–369. [Google Scholar]
- Davyt, D; Fernandez, R; Suescun, L; Mombrú, AW; Saldaña, J; Domínguez, L; Coll, J; Fujii, MT; Do Manta, E. New sesquiterpene derivatives from the red alga Laurencia scoparia. Isolation, structure determination, and anthelmintic activity. J. Nat. Prod 2001, 64, 1552–1555. [Google Scholar]
- Topcu, G; Aydogmus, Z; Imre, S; Gören, AC; Pezzuto, JM; Clement, JA; Kingston, DGI. Brominated sesquiterpenes from the red alga Laurencia obtusa. J. Nat. Prod 2003, 66, 1505–1508. [Google Scholar]
- Juagdan, EG; Kalidindi, R; Scheuer, P. Two new chamigranes from an hawaiian red alga, Laurencia cartilaginea. Tetrahedron 1997, 53, 521–528. [Google Scholar]
- Sun, J; Shi, D; Ma, M; Li, S; Wang, S; Han, L; Yang, Y; Fan, X; Shi, J; He, L. Sesquiterpenes from the red alga Laurencia tristicha. J. Nat. Prod 2005, 68, 915–919. [Google Scholar]
- Kladi, M; Vagias, C; Papazafiri, P; Brogi, S; Tafi, A; Roussis, V. Tetrahydrofuran acetogenins from Laurencia glandulifera. J. Nat. Prod 2009, 72, 190–193. [Google Scholar]
- Kladi, M; Vagias, C; Stavri, M; Rahman, M; Gibbons, S; Roussis, V. C15 acetogenins with antistaphylococcal activity from the red alga Laurencia glandulifera. Phytochem. Lett 2008, 1, 31–36. [Google Scholar]
- Lhullier, C; Donnangelo, A; Caro, M; Palermo, JA; Horta, PA; Falkenberg, M; Schenkel, EP. Isolation of elatol from Laurencia microcladia and its palatability to the sea urchin Echinometra lucunter. Biochem. Syst. Ecol 2009, 37, 254–259. [Google Scholar]
- Ji, N-Y; Li, X-M; Li, K; Wang, B-G. Halogenated sesquiterpenes from the marine red alga Laurencia saitoi (Rhodomelaceae). Helv. Chim. Acta 2009, 92, 1873–1879. [Google Scholar]
- Pettit, GR; Herald, CL; Allen, MS; Von Dreele, RB; Vanell, LD; Kao, JPY; Blake, W. The isolation and structure of aplysistatin. J. Am. Chem. Soc 1977, 99, 262–263. [Google Scholar]
- Pandey, PK. Endangered medicinal species of the Indian Ocean: radical need for conservation. Chem. Biodivers 2009, 6, 990–1001. [Google Scholar]
- Su, H; Yuan, Z; Li, J; Guo, S; Han, L; Zhu, X; Shi, D. Studies on chemical constituents of Laurencia saitoi. Zhongguo Zhong Yao Za Zhi 2009, 34, 871–874. [Google Scholar]
- Su, H; Yuan, Z-H; Li, J; Guo, S-J; Deng, L-P; Han, L-J; Zhu, X-B; Shi, D-Y. Sesquiterpenes from the marine red alga Laurencia saitoi. Helv. Chim. Acta 2009, 92, 1291–1297. [Google Scholar]
- Afolayan, AF; Mann, MGA; Lategan, CA; Smith, PJ; Bolton, JJ; Denzil, R; Beukes, DR. Antiplasmodial halogenated monoterpenes from the marine red alga Plocamium cornutum. Phytochemistry 2009, 70, 597–600. [Google Scholar]
- Lane, AL; Stout, EP; Lin, A-S; Prudhomme, J; Le Roch, K; Fairchild, CR; Franzblau, SG; Hay, ME; Aalbersberg, W; Kubanek, J. Antimalarial bromophycolides J-Q from the fijian red alga Callophycus serratus. J. Org. Chem 2009, 74, 2736–2742. [Google Scholar]
- Areche, C; San-Martín, A; Rovirosa, J; Soto-Delgado, J; Contreras, R. An unusual halogenated meroditerpenoid from Stypopodium flabelliforme: Studies by NMR spectroscopic and computational methods. Phytochemistry 2009, 70, 1315–1320. [Google Scholar]
- Reddy, P; Urban, S. Meroditerpenoids from the southern Australian marine brown alga Sargassum fallax. Phytochemistry 2009, 70, 250–255. [Google Scholar]
- Ji, N-Y; Wen, W; Li, X-M; Xue, Q-Z; Xiao, H-L; Wang, B-G. Brominated selinane sesquiterpenes from the Marine Brown Alga Dictyopteris divaricata. Mar. Drugs 2009, 7, 355–360. [Google Scholar]
- Cardozo, KHM; Guaratini, T; Barros, MP; Falcão, VR; Tonon, AP; Lopes, NP; Campos, S; Torres, MA; Anderson, O; Souza, AO; Colepicolo, P; Pinto, E. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C: Toxicol. Pharmacol 2007, 146, 60–78. [Google Scholar]
- Tan, LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar]
- Borowitzka, MA. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol 1995, 7, 3–15. [Google Scholar]
- Burja, AM; Banaigs, B; Abou-Mansour, E; Burgess, JG; Wright, PC. Marine cyanobacteria-a prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar]
- Kwan, JC; Rocca, JR; Abboud, KA; Paul, VJ; Luesch, H. Total structure determination of grassypeptolide, a new marine cyanobacterial cytotoxin. Org. Lett 2008, 10, 789–792. [Google Scholar]
- Luesch, H; Yoshida, WY; Moore, RE; Paul, VJ; Corbett, TH. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc 2001, 123, 5418–5423. [Google Scholar]
- Taori, K; Paul, VJ; Luesch, H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J. Am. Chem. Soc 2008, 130, 1806–1807. [Google Scholar]
- Paerl, HW; Huisman, J. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep 2009, 1, 27–37. [Google Scholar]
- Jiménez, JI; Vansach, T; Yoshida, WY; Sakamoto, B; Pörzgen, P; Horgen, FD. Halogenated fatty acid amides and cyclic depsipeptides from an eastern caribbean collection of the cyanobacterium Lyngbya majuscule. J. Nat. Prod 2009, 72, 1573–1578. [Google Scholar]
- Kwan, JC; Taori, K; Paul, VJ; Luesch, H. Lyngbyastatins 8–10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium Lyngbya semiplena. Mar. Drugs 2009, 7, 528–538. [Google Scholar]
- Mo, S; Krunic, A; Chlipala, G; Orjala, J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod 2009, 72, 894–899. [Google Scholar]
- Volk, R-B; Girreser, U; Al-Refai, M; Laatsch, H. Bromoanaindolone, a novel antimicrobial exometabolite from the cyanobacterium Anabaena constricta. Nat. Prod. Res 2009, 23, 607–612. [Google Scholar]
- Hayes, KF; Millar, JG. Methods in Chemical Ecology: Bioassay Methods; Kluwer Academic: Norwell, MA, USA, 1998. [Google Scholar]
- Millar, JG; Hayes, KF. Methods in Chemical Ecology: Chemical Methods; Kluwer Academic: Norwell, MA, USA, 1998. [Google Scholar]
- Plaza, M; Santoyo, S; Jaime, L; García-Blairsy Reina, G; Herrero, M; Señoráns, FJ; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal 2010, 51, 450–455. [Google Scholar]
- Prince, EK; Pohnert, G. Searching for signals in the noise: metabolomics in chemical ecology. Anal. Bioanal. Chem 2010, 396, 193–197. [Google Scholar]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modeling to understand metabolic networks. Compar. Funct. Genom 2001, 2, 155–168. [Google Scholar]
- Bundy, JG; Davey, MP; Viant, MR. Environmental metabolomics: a critical review and future perspectives. Metabolomics 2009, 5, 3–21. [Google Scholar]
- Barofsky, A; Vidoudez, C; Pohnert, G. Metabolic profiling reveals growth stage variability in diatom exudates. Limnol. Oceanogr. Meth 2009, 7, 382–390. [Google Scholar]
- Nyadong, L; Hohenstein, EG; Galhena, AS; Lane, AL; Kubanek, J; Sherrill, CD; Fernandez, FM. Reactive desorption electrospray ionization mass spectrometry (DESI-MS) of natural products of a marine alga. Anal. Bioanal. Chem 2009, 394, 245–254. [Google Scholar]
- Lane, AL; Nyadong, L; Galhena, AS; Shearerb, TL; Stout, EP; Parry, RM; Kwasnik, M; Wang, MD; Hay, ME; Fernandez, FM; Kubanek, J. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc. Natl. Acad. Sci. USA 2009, 106, 7314–7319. [Google Scholar]
- Wiseman, JM; Laughlin, BC. Desorption electrospray ionization (DESI) mass spectrometry: a brief introduction and overview. Curr. Sep. Drug Dev 2007, 22, 11–14. [Google Scholar]
- Cooks, RG; Ouyang, Z; Takats, Z; Wiseman, JM. Ambient mass spectrometry. Science 2006, 311, 1566–1570. [Google Scholar]
- Lobban, CS; Harrison, PJ. Seaweed Ecology and Physiology; Cambridge University Press: New York, NY, USA, 1994. [Google Scholar]
- Amsler, CD; Iken, K; McClintock, JB; Baker, BJ. Defenses of polar macroalgae against herbivores and biofoulers. Bot. Mar 2009, 52, 535–545. [Google Scholar]
- Hay, ME; Fenical, W. Chemical ecology and marine biodiversity: insights and products from the sea. Oceanography 1996, 9, 10–19. [Google Scholar]
- Lane, AL; Nyadong, L; Galhena, AS; Shearer, TL; Stout, EP; Parry, RM; Kwasnik, M; Wang, MD; Hay, ME; Fernandez, FM; Kubanek, J. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc. Natl. Acad. Sci. USA 2009, 106, 7314–7319. [Google Scholar]
- Norse, EA. Global Marine Biological Diversity: A Strategy for Building Decision into Decision Making; Island Press: Washington DC, USA, 1993. [Google Scholar]
- Paul, VJ; Arthur, KE; Ritson-Williams, R; Ross, C; Sharp, K. Chemical defenses: from compounds to communities. Biol. Bull 2007, 213, 226–251. [Google Scholar]
- Nagai, H; Murata, M; Torigoe, K; Satake, M; Yasumoto, T. Gambieric acids: new potent antifungal substances with unprecedented polyether structures from a marine dinoflagellate Gambierdiscus toxicus. J. Org. Chem 1992, 57, 5448–5453. [Google Scholar]
- Capon, RJ; Barrow, RA; Rochfort, S; Jobling, M; Skene, C. Marine nematodes: tetrahydrofurans from a Southern Australian brown alga Notheia anomala. Tetrahedron 1998, 54, 2227–2242. [Google Scholar]
- Etahiri, S; Bultel-Ponce, V; Caux, C; Guyot, M. New bromoditerpenes from the red alga Sphaerococcus coronopifolius. J. Nat. Prod 2001, 64, 1024–1027. [Google Scholar]
- Sakemi, S; Higa, T; Jefford, CW; Bernardinelli, G. Venustatriol, a new anti-viral triterpenes tetracyclic ether from Laurencia venusta. Tetrahedron Lett 1986, 27, 4287–4290. [Google Scholar]
- Pohnert, G; Steinke, M; Tollrian, R. Chemical cues, defense metabolites and the shaping of pelagic interspecific interactions. Trends Ecol. Evol 2007, 22, 198–204. [Google Scholar]
- Amsler, CD. Amsler, CD, Ed.; Algal sensory chemical ecology. In Algal Chemical Ecology; Springer: Berlin, Heidelberg, Germany, 2008; pp. 297–309. [Google Scholar]
- Poulson, KL; Sieg, RD; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep 2009, 26, 729–745. [Google Scholar]
- Prince, EK; Myers, TL; Naar, J; Kubanek, J. Competing phytoplankton undermines allelopathy of a bloom-forming dinoflagellate. Proc. R. Soc. B 2008, 275, 2733–2741. [Google Scholar]
- Tameishi, M; Yamasaki, Y; Nagasoe, S; Shimasaki, Y; Oshima, Y; Honjo, T. Allelopathic effects of the dinophyte Prorocentrum minimum on the growth of the bacillariophyte Skeletonema costatum. Harmful Algae 2009, 8, 421–429. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated Compounds from Marine Algae. Mar. Drugs 2010, 8, 2301-2317. https://doi.org/10.3390/md8082301
Cabrita MT, Vale C, Rauter AP. Halogenated Compounds from Marine Algae. Marine Drugs. 2010; 8(8):2301-2317. https://doi.org/10.3390/md8082301
Chicago/Turabian StyleCabrita, Maria Teresa, Carlos Vale, and Amélia Pilar Rauter. 2010. "Halogenated Compounds from Marine Algae" Marine Drugs 8, no. 8: 2301-2317. https://doi.org/10.3390/md8082301



