2.2. Drug Excipient Compatibility
Differential scanning calorimetry (DSC) was used as a technique to measure the compatibility of the drugs with chitin-metal silicate (CMS) by measuring the physical transformation of the drugs when they undergo phase transitions due to endothermic or exothermic heat flows. Examples of such thermograms are presented in
Figures 1 and
2.
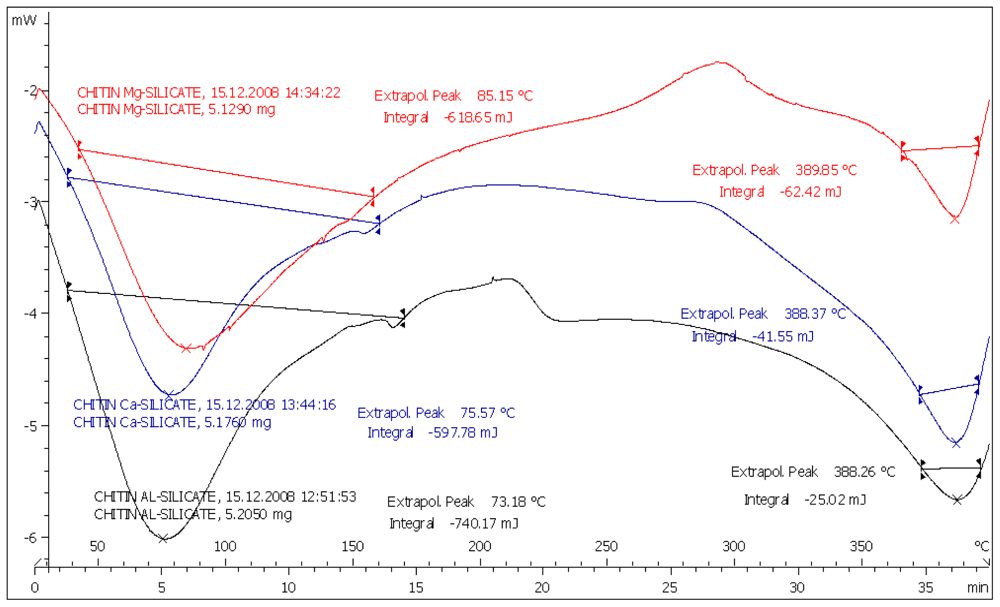
All the CMS(s) showed broad spectrum endothermic peaks at 30–120 °C (
Figure 1). Many authors [
13] related the presence of such broad spectrum peak in this region to water evaporation of polymeric materials.
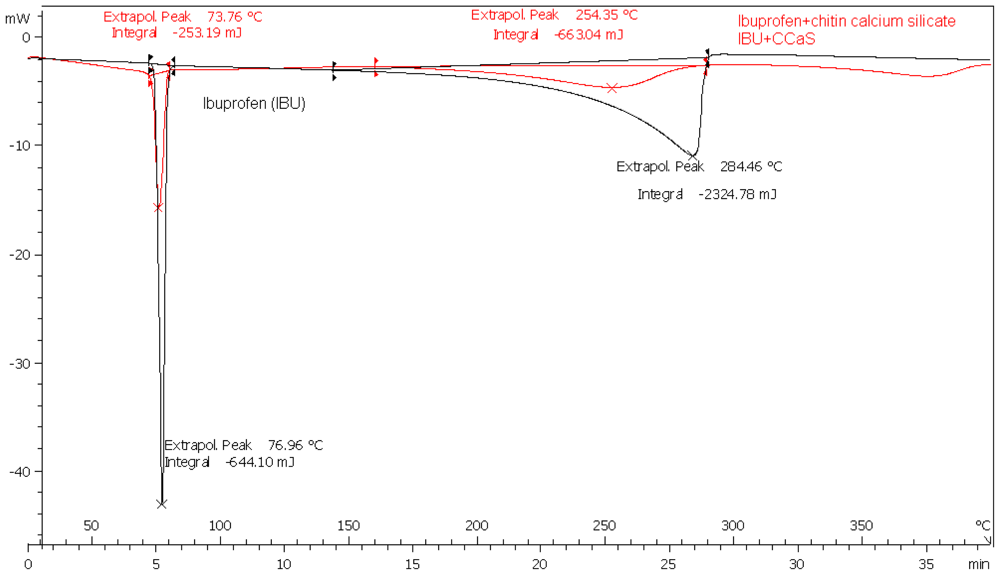
Sharp endothermic peaks due to melting at 76, 160 and 210 °C were recorded for IBU, MET and SPL, respectively. Endothermic peaks shown above 250 °C are due to the decomposition of chitin itself. The melting endotherms of the physical mixture of the drugs with CMS were well preserved with slight changes in terms of broadening or shifting towards lower temperatures (Figures for MET and SPL are not shown). It has been reported that the quantity of material used, especially in drug-excipient mixtures, affects the peak shape and enthalpy. Thus, these changes in the melting endotherm could be due to the mixing process, which lowers the purity of each component in the mixture, and may not necessarily indicate potential chemical incompatibility [
14]. However, as the physical interaction may affect the crystalline structure, this may be correlated to the observed lowering in the melting endotherms. Such results indicate no chemical interaction is detectable.
2.3. Compression Studies Analysis
The compression behavior of the three model drugs, CMgS, and mixture of CMgS with the drugs were analyzed using the Heckel equation (Equation 4).
Powders of MET and SPL failed to form solid compacts at the pressures applied (2.7–44.2 MPa), while IBU was compressed easily over this pressure range. However, it was not possible to obtain Heckel plots since these powders form non porous solid that formed a low volume tablet.
Heckel plots (not shown) of CMgS, CMgS with IBU, MET, or SPL showed pronounced initial curvature followed by a linear portion. This is an indicator of intensive particle rearrangements and fragmentation followed by plastic deformation. All tested formulas showed similar Heckel plots, which mean that the addition of drugs did not affect the shape of the Heckel plot. Different parameters were obtained from Heckel plots and are presented in
Table 1. The
Py values, which represent the yield pressure (
i.e., the least pressure at which particle plastic deformation is initiated), decreased from 153.9 MPa; for the single excipient,
i.e., chitin Mg silicate (CMgS), to 82, 86.3, and 103.1 MPa for the mixtures of the same excipient with IBU, MET and SPL drugs, respectively (calculated
p < 0.05 of the yield pressure values between that of the single excipient and that of the excipient with the incorporated drugs). This implies that, the onset of plastic deformation of CMgS occurred at lower pressure in the presence of drugs [
15]. This implies that CMgS can be useful in high-speed tablet machines with short dwell time, where faster onset of plastic deformation should be useful in avoiding problems of lamination and capping [
16].
The D0 value, which represents the degree of initial packing in the die as a result of die filling for CMgS, increased in the presence of drugs and were in the order: CMgS with IBU > CMgS with MET > CMgS with SPL > CMgS. This shows that the CMgS/drug mixtures exhibit higher degree of packing in the dies as a result of die filling than CMgS powder alone.
The value of DB represents the phase of rearrangement of the particles in the early stages of compression. The DB value of CMgS powder alone was 0.36 and increased to 0.44, 0.48, 0.49 for CMgS with IBU, CMgS with MET, and CMgS with SPL, respectively. This increase indicates that the fragmentation of the powder mixture is more pronounced than the CMgS alone. The value of DA, which represents the total degree of packing achieved at zero and low pressure (i.e., D0 + DB), increased for CMgS in the presence of drugs.
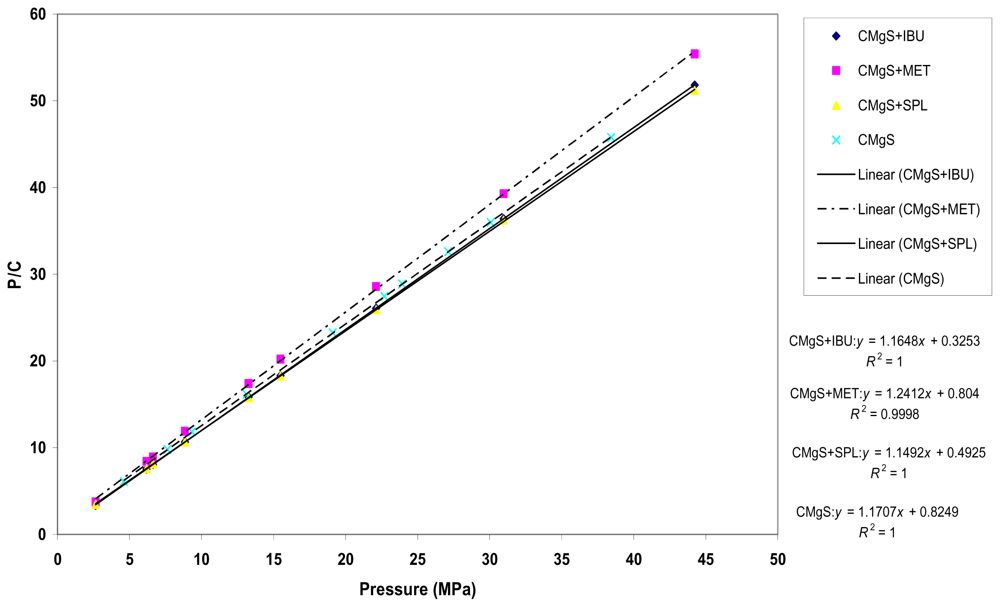
Figure 3 showed Kawakita plots for CMgS alone and CMgS with IBU, MET and SPL, respectively. A linear relationship between
P/C and
P was obtained at all applied compression pressures with a correlation coefficient ≥ 0.99. Values of “
1/a” and “
1/ab” were obtained from the slope and intercept of the plots, respectively, and are summarized in
Table 2.
From the table, the value of “a” parameter, which represents the compressibility (total volume reduction) of the powder before compression, for CMgS alone was not significantly different (p > 0.05) from the “a” values of CMgS with the drugs. This means that the addition of a drug to the excipient did not significantly alter the measured compressibility of the powder.
The value of “1/b” for CMgS was higher than CMgS with drugs. The lower value of “1/b” of CMgS in the presence of drugs is indicative of the reduction in cohesive forces. In other words, the drugs increased the total degree of plastic deformation of CMgS during the compression process. These results positively correlate with Heckel parameter “Py”.
2.4. Physical Properties of the Tablets
The physical properties of all the tablet formulations are presented in
Table 3. All tablets passed the current USP friability test, showing the % friability value well below the 1% upper level of acceptability for pharmaceutical tablets (USP, 2007). The formulated tablets showed thicknesses that are comparable to their respective commercial products. Acceptable content uniformity of the produced tablets was obtained ranging from 98 ± 2 to 101 ± 2%. Regarding the crushing strength, the results are in good agreement with the friability data and are considered to be acceptable values (>40 N) [
17]. The higher thickness values for tablets made using CMS compared to that of Avicel
® 200 tablets is due to the high elasticity of these material.
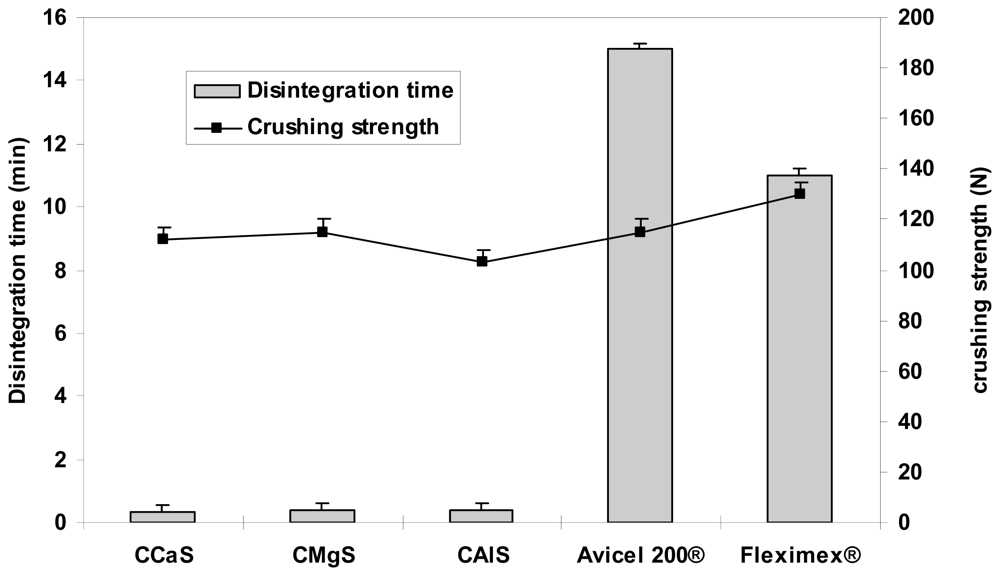
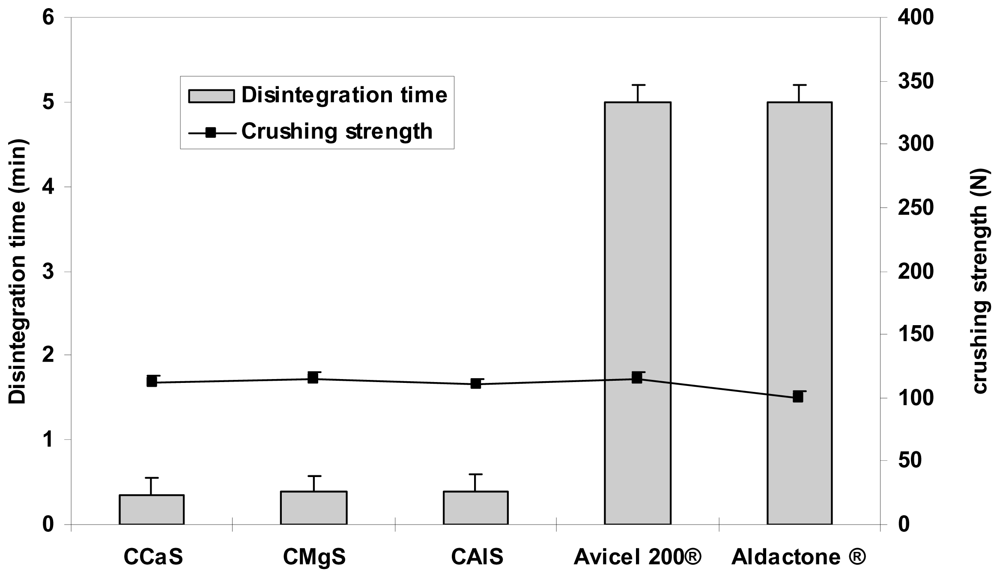
Figures 4–
6 represent the crushing strengths and the disintegration times of the tablets prepared by direct compression using CMS in comparison with Avicel
® 200 (one of the most commercially used excipient in direct compression) and the respective commercial products. The crushing strengths of all tablet formulation were within 100–115 N. No significant difference (
p > 0.05) in the crushing strengths was found between the three CMS. It showed superdisintegration properties. The three drug formulations disintegrated in less than one minute (
p > 0.05). On the other hand, Avicel
® 200 tablets disintegrated in 15 min, less than one minute, and 5 min for IBU, MET, and SPL tablets, respectively.
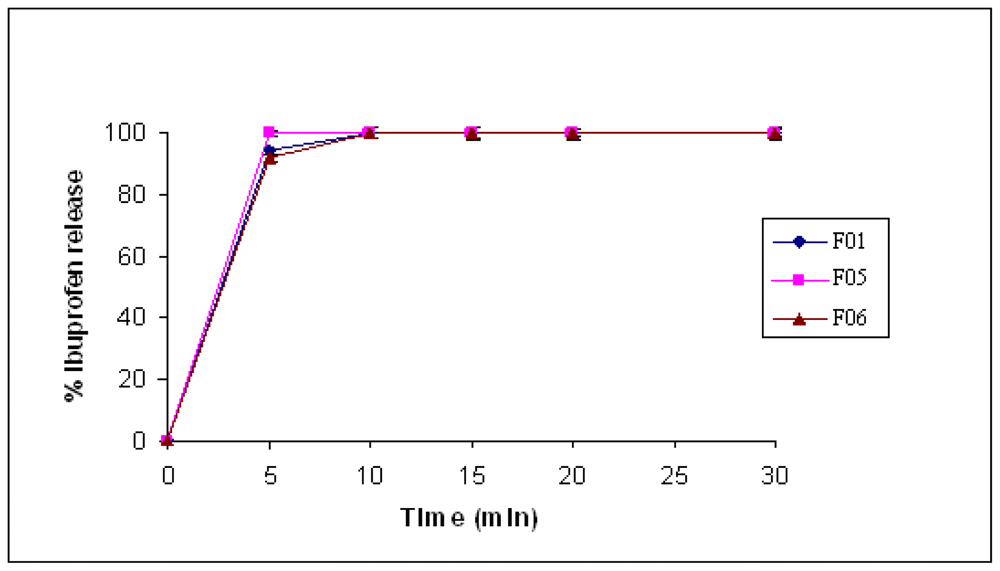
The CMgS was used as intra-granular as well as extra-granular in the preparation of IBU and MET tablet formulations using the wet granulation method. The crushing strength and disintegration time results obtained are summarized in
Table 4.
From the above results it can be concluded that intra-granular method resulted in harder tablets than extra-granular method, and this could be due to the binding effect of water used in the granulation. Intra-granulation enhances distribution of the inactive material (CMgS) in closer fashion around the active particles, enhancing bond formation between the active and inactive materials, which both form a single entity as a result of drying. The superdisintegration property of CMgS was not lost due to wet granulation; the tablets disintegrate in less than one minute. Such results enforce the idea that this multifunctional excipient can be used in dry and wet granulation with preferential addition as intragranulation method.
2.5. Drug Release Studies
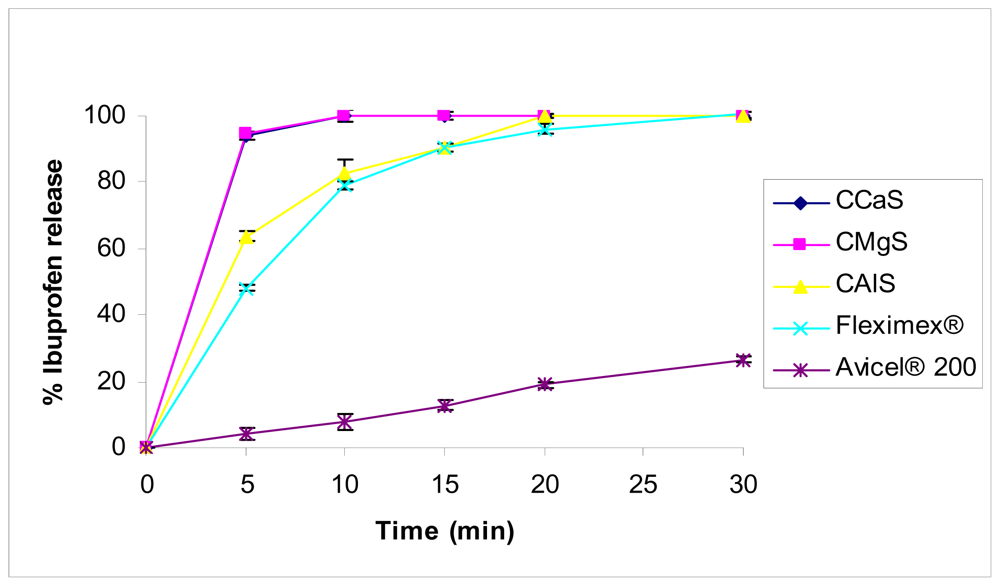
For IBU tablets,
Figure 7, the CMgS and CCaS (Formulas F1 and F2, respectively) released 94% of the drug in 10 min while 84% of IBU was released from CAlS tablets (Formula F3) within the same time period. 100% of the drug was released from CMgS and CCaS tablets in 10 min, while 20 min and 30 min was required to release the same amount of the drug from CAlS and Fleximex
® tablets, respectively. This criterion of dissolution fulfills the USP criteria for immediate release solid dosage forms. Avicel
® 200, in contrast, released only 25% of the drug in 30 min. CalS, which is considered to be acidic filler, resulted in an acidic pH-microenvironment around the acidic IBU particles. This could be the reason behind the slower release profile compared to the basic fillers CcaS- and CMgS-containing tablets. In a similar study, [
17] used cellulose II powder as a single filler excipient with multi functional action. Drug release from the tablets containing cellulose II powder was faster than both the commercial product (Advil
®) and Avicel
® pH 102 containing tablets. They related this observation to the faster disintegration of cellulose II containing tablets. It seems that the use of a single excipient composed of chitin-Mg, Ca silicate mixtures can be used alone to yield an immediate release tablet of IBU similar to what has been reported.
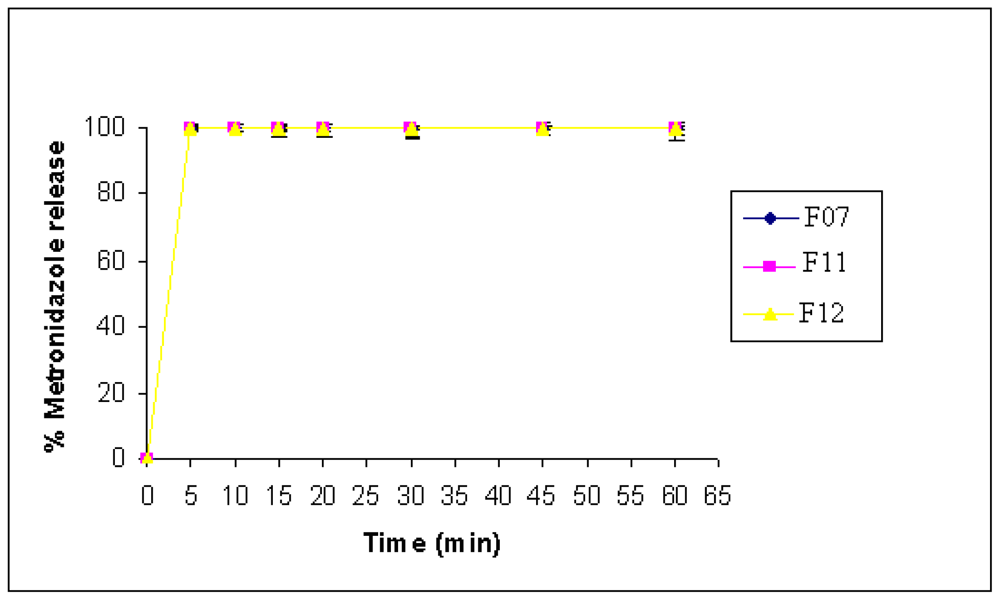
The effect of the type of CMS was not significantly noticed on MET, since MET is highly soluble in the dissolution medium used. Complete MET release was within 5 min from all types of CMS. These results reveal that all tablet formulations met the USP drug release tolerance criterion.
For SPL tablets, preliminary data using release studies using the USP conditions (1000 mL of 0.1 N HCl containing 0.1% sodium lauryl sulfate, Paddle, 75 rpm) doesn’t indicate any significant difference (
p > 0.05) between the commercial product and CMS containing tablets. This could be attributed to the presence of hydrochloric acid as an acidic medium that could level off the micro-environmental pH differences between different metal silicates present in the CMS tablets. Consequently, water was used as a dissolution medium in order to get an insight about the differences in the microenvironment pH and its effect on drug release. The comparative release profiles of the water insoluble drug, SPL, are presented in
Figure 8. The release rate decreased in the order: Aldactone
® > CMgS ~ CAlS ~ CCaS > Avicel
® 200. All tablet formulations made using CMS showed negligible difference in the dissolution profile (p < 0.05). Certainly, CMS type did not affect the dissolution profile of SPL, since it is a neutral drug. The CMS containing formulas showed a significant improvement in drug dissolution rate compared to Avicel
® 200 containing formula. However, the market formulation of SPL, Aldactone
®, showed a faster dissolution rate than CMS made formula, this could be due to the many excipients included in Aldactone
® (such as calcium sulfate, corn starch, hypromellose, polyethylene glycol, povidone) [
18,
19]. SPL is water insoluble drug and to have a dissolution profile comparable with the leading brand is a real advance in excipient technology, particularly because its formulation is only containing the present multifunctional excipient.