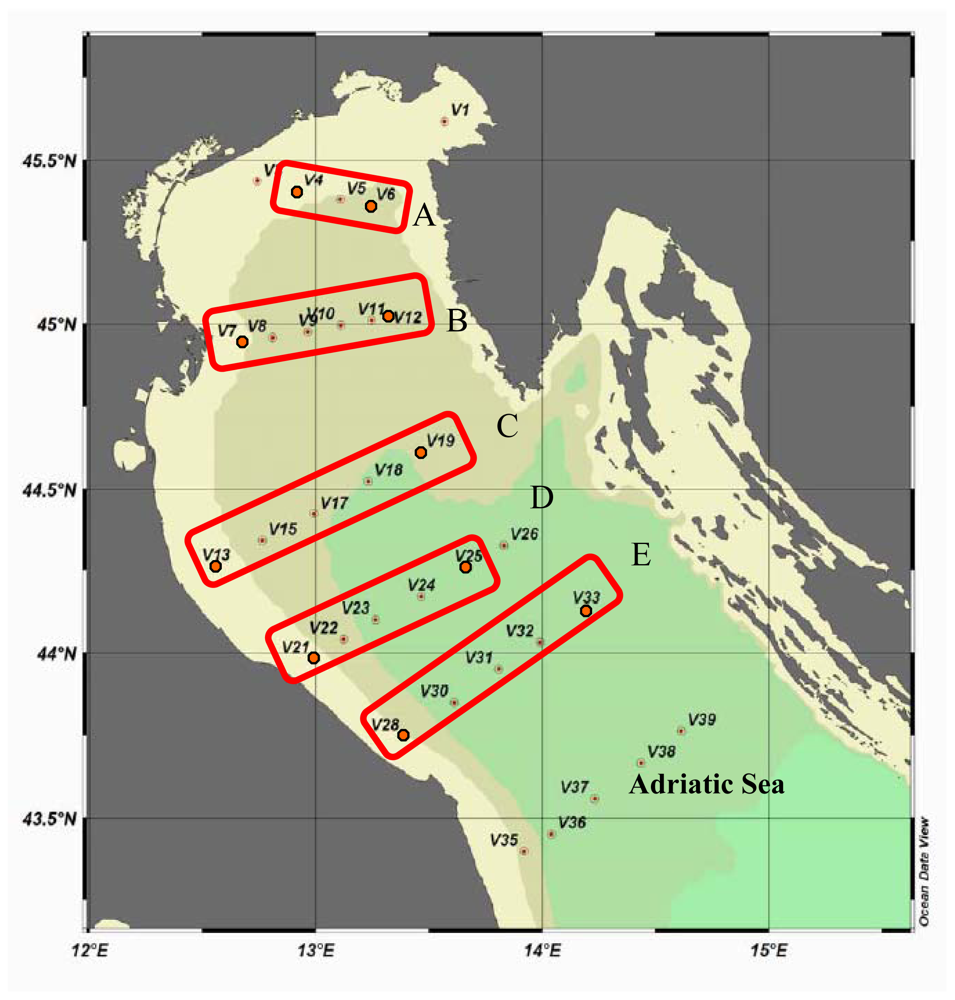
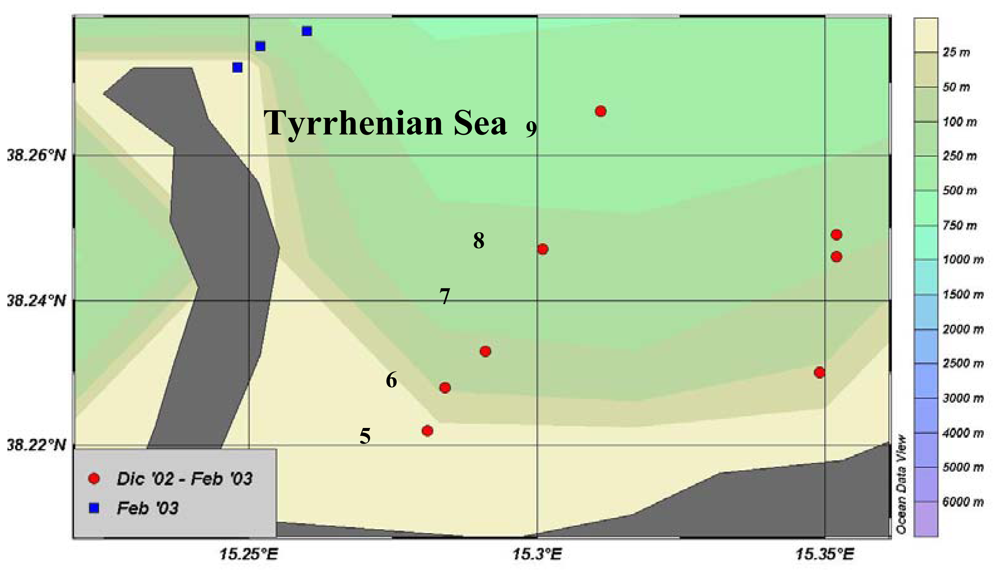
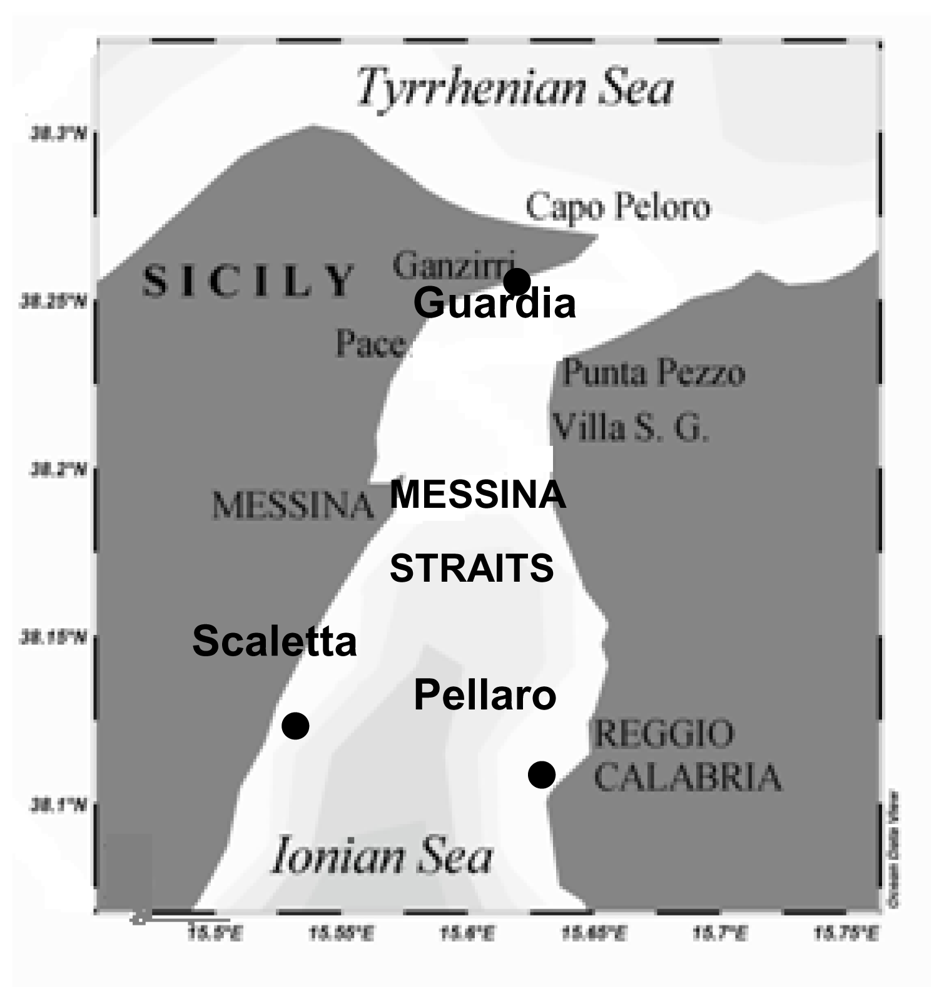
2.1. Northern Adriatic Basin
During February 2008, the Adriatic waters displayed temperatures ranging from 7.76 to 11.68 °C and salinity values varied between 34.32 and 38.55.
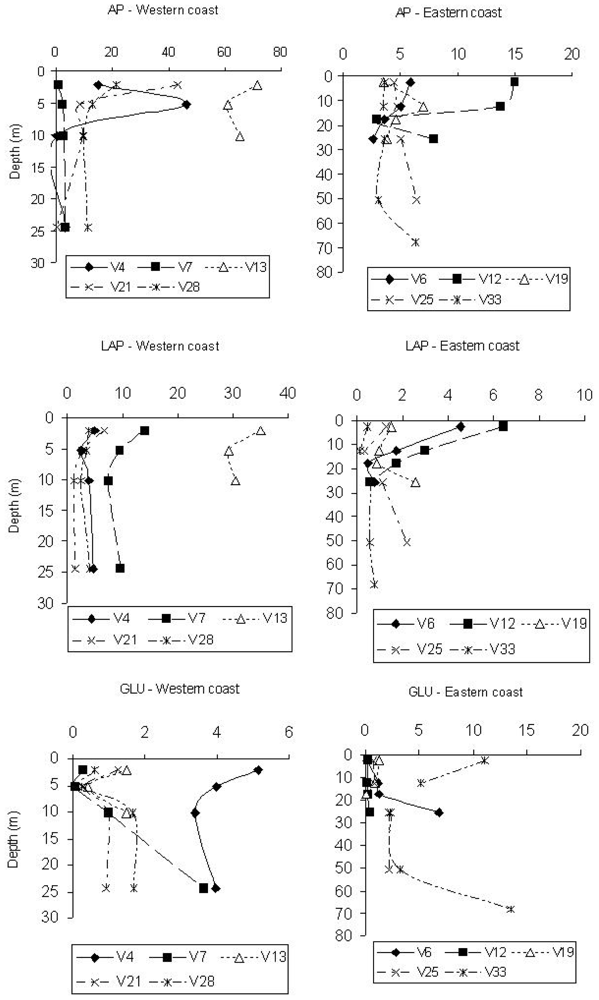
Table 1 shows the mean value and ranges of variation of enzyme activity rates. Enzyme patterns showed high AP activity rates; discrete values of LAP and β-GLU were also found.
Spatially, maximum AP values were found at stations V13 and V4, belonging to transects C and A, respectively. In contrast, minimum values were detected at station V7, belonging to transect B. The Eastern side was characterised by AP activity rates on average ¼ lower than those measured along the Italian coast (
Figure 1). AP activity levels decreased south of the Po river, suggesting a lower amount of organic phosphates or the availability of P.
Vertical AP profiles (
Figure 1) showed higher enzyme values at surface or sub-surface depths, such as at 5 m at station V4; some activity peaks, however, were found at the bottom of the stations V25 and V33, along the Eastern coast.
LAP rates were higher on the Western Adriatic side, especially at stations V13 and V7, and lower at stations V21 and V28 (transects D and E). The Eastern stations displayed enzyme levels about 1/3 lower than those measured at the Western ones; minimum values were found at station V33 (
Figure 1). LAP levels were related to organic matter inputs coming from the Po river; in fact, station 13 was characterised by the highest mean POC and Chl-
a values (41.54 and 1.45 μg/L, respectively), while the lowest ones were found at station 33 (3.48 and 0.152 μg/L, respectively).
Integrated LAP values obtained for each station (not reported in figure) showed that enzyme levels followed an increasing pattern from station V33 northwards to station V12 (0.507 to 2.943 μmol/m3/h) along the Eastern side, and from station V4 southwards to station V13 (4.01 to 31.50 μmol/m3/h) along the Western side, in agreement with the circulation pattern of the water masses within the Northern Adriatic basin.
Vertical LAP profiles (
Figure 1) showed the decrease of enzyme activity levels from surface to bottom along the coastal side affected by the Po river inputs, while at stations V19, V25 and V33, located along the Eastern coast, peaks at greater depths were observed. Here, at the bottom, POC values were slightly higher than those found in the nearest upper layers (3.82, 3.63, 3.12 μg/L
vs. 3.32, 3.08 and 1.76 μg/L, respectively), suggesting the sinking of labile matter.
The highest β-GLU activity rates were measured at stations V33 (transect E), V4 and V6 (transect A); in contrast, minimum values were detected at station V12 (Eastern side) (
Figure 1). The highest activity rates were found along the Western side; particularly, at station V4 the lowest amounts of Chl-
a and POC were measured (0.090 and 3.14 μg/L, respectively). From the integrated β-GLU values obtained for each station (not shown), a decreasing gradient was observed from station V4 southwards to station V13 (4.117 to 1.130 μmol/m
3/h) on the Western coast, and from station V33 northwards to station V12 (7.055 to 0.271 μmol/m
3/h) on the Eastern one. This latter side was characterised by enzyme values about 1.3 times higher than those measured on the Western one.
Vertical β-GLU profiles (
Figure 1) mostly followed an increasing trend with depth; generally, at the Eastern side stations a higher activity was found at lower depths (stations V6 and V33), suggesting the presence of more refractory substrates, probably of polysaccharidic origin. This was also in agreement with the detection at station 33 of the lowest concentrations of Chl-
a, as an indicator of fresh organic matter.
Pooling all the data collected from the Western (n = 20) and the Eastern stations (n = 22) separately, AP rates were negatively affected by salinity (r = −0.811, P < 0.01) along the Western side; by temperature (r = −0.684, P < 0.01) along the Eastern one. LAP activity rates showed negative correlations with salinity (r = −0.771, P < 0.01) along the Po coast as well as along the Eastern side (r = −0.833, P < 0.01). LAP correlated inversely with temperature (r = −0.799, P < 0.01) along the Eastern side. No significant correlations were calculated between β-GLU and temperature or salinity. On the Western side, the statistical relationships linking enzyme activity rates with POC and Chl-a concentrations were generally more significant (for LAP: r = 0.901 and 0.972; for AP: r = 0.921 and 0.952, respectively) than those found on the Eastern one (for LAP: r = 0.904 and 0.832; for AP: r = 0.784 and 0.729, respectively).
2.5. Main Considerations on Enzyme Patterns and Their Biogeochemical Significance
Microbial assemblages play a dominant role in the transformation and mineralization of organic matter in the marine environment [
1,
6,
9]. Dissolved organic polymers are the major reservoirs of Carbon and nutrients in the waters; while they are produced by different planktonic sources and biological processes, as well as introduced from land and fluvial inputs, they are consumed and recycled mainly through bacterioplankton. Therefore, bacterial metabolism regulates the cycling of biogenic elements [
31]. Before the present study, the patterns of microbial activities involved in biogeochemical cycles were fully unknown in the waters of the Gulfs of Manfredonia and Milazzo, as well as in the Straits of Messina. The enzyme measurements reported here are the first available for these coastal areas, in contrast to some other Mediterranean sites (Ligurian Sea [
23]; northwestern Mediterranean, DYFAMED station located at the western part of the Ligurian Sea [
21,
22,
24,
25]), where extensive studies were previously performed. Even in the Northern Adriatic basin, where most of the studies dealt with the appearance of mucilages [
17,
20,
32,
33], only a few investigations focused on the dynamics of microbial assemblage (abundance and activity) on a wide spatial (
i.e., basin) scale similar to that considered in this study.
The use of fluorogenic substrates to estimate enzyme activity rates provide a “potential” estimate of activity only; nevertheless, this potential information is of great ecological significance, as it contributes to knowledge of the microbial potentialities on the organic matter pool and therefore to better define the role played by microorganisms in the functioning of aquatic ecosystems.
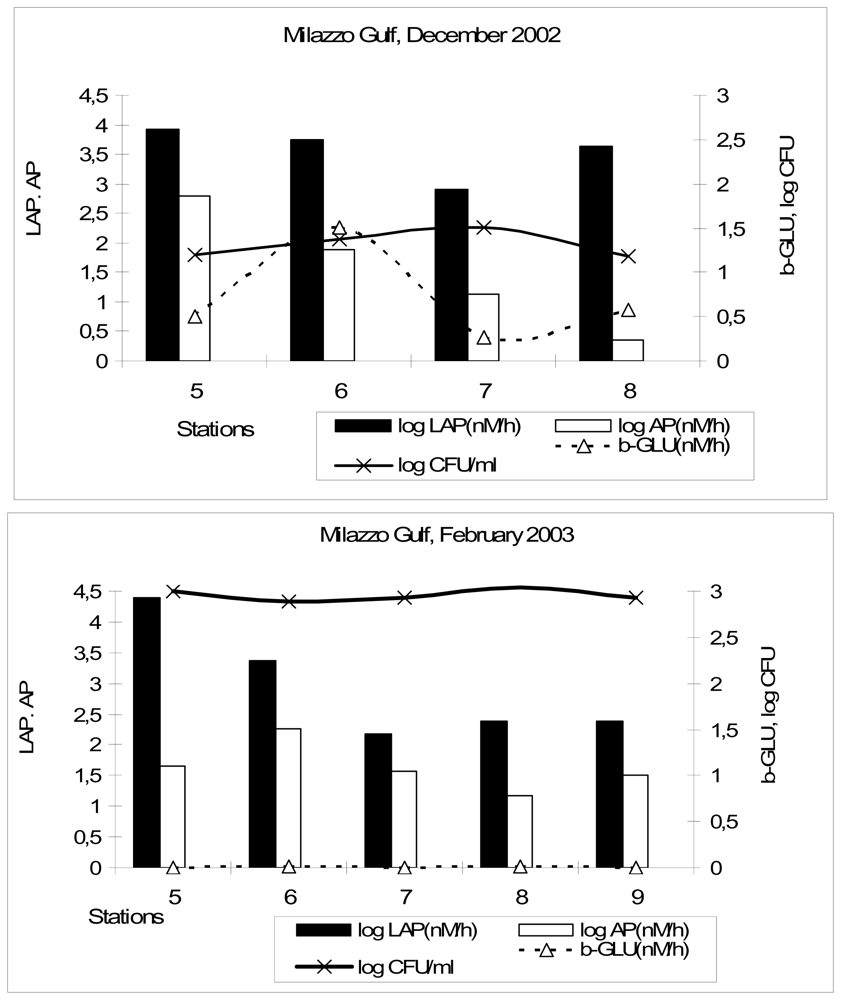
The measured enzyme activity rates, converted into nanograms of Carbon and Phosphorus potentially released from the substrates, are summarised as the mean values obtained for each area and reported in
Table 5. LAP and AP values increased from the Northern Adriatic Sea to the Straits of Messina, through the Gulf of Manfredonia; β-GLU was at the lowest levels in the Gulf of Milazzo (February 2003). Enzyme values detected in the study areas fall within a range similar to that detected in other Mediterranean coastal environments [
1,
15,
16,
19,
21,
32,
33].
This comparative investigation pointed out the widespread occurrence of LAP and AP activities in the studied coastal areas, confirming the high potential of the microbial community to degrade preferentially proteins and organic phosphoric esters, respectively. In the waters of the North Adriatic Sea, this result agreed with the
in vitro determination of enzyme profiles, which showed the expression of peptidase and phosphatase by a large (60%) percentage of bacterial isolates in all the seasons [
17].
The microbial enzyme activity patterns found in this study were characterised by high variability both in space and time. Analysis of variance (ANOVA) revealed significant spatial differences among the different ecosystems in terms of their LAP, β-GLU and AP values (F: 29.74, 52.72, 5.11, P < 0.01, respectively).
The values of Michaelis-Menten constant (K
m), reported in
Table 5, suggested a significantly (p < 0.01) lower substrate affinity of LAP in the Gulf of Milazzo (February 2003) compared to the highest affinity measured in the Gulf of Manfredonia. Conversely, the Gulf of Milazzo (February 2003) was characterised by a much higher substrate affinity of β-GLU with respect to that measured in the Straits of Messina. A low K
m value found for AP in the Straits of Messina indicated that in this area the microbial community could use a small amount of substrate effectively, while the high K
m found in the Gulf of Milazzo (December 2002) suggested the need for high substrate concentration to achieve maximum reaction velocity. High substrate affinity is considered a strategy to support bacterial growth under low nutrient conditions [
34].
The enzymatic ability measured in coastal environments allows to estimate the amount of nutrients that can be potentially mobilised and exported to the open sea. The potential remineralisation rate of phosphorus by AP was 9.43 μg P/dm3/day in the Northern Adriatic Sea and 26.34 μg P/dm3/day in the Gulf of Manfredonia; the same enzyme activity was estimated to contribute to the release of 80.34 μg P/dm3/day in the Straits of Messina, taking into account the entire period. In the Gulf of Milazzo, AP activity potentially contributed to the remineralisation of 182.1 and 46.51 μg P/dm3/day in December and in February, respectively.
LAP potentially accounted for the release of 1.76 μg of dissolved organic N/dm3/day in the Northern Adriatic Sea, while this amount increased in the Gulf of Manfredonia and in the Straits of Messina, where we estimated that the dissolved organic N potentially mobilised per day was 6.55 μg and 17.27 μg N/dm3/day, respectively. In the Gulf of Milazzo, LAP activity potentially released 1.80 μg and 1.87 mg of dissolved organic N/dm3/day in December and in February, respectively.
Comparing the different ecosystems, the highest potential mineralization rate of P by AP, calculated on a daily scale, was found in the Gulf of Milazzo (December 2002) and in the Straits of Messina; LAP potentially contributed to the release of high amounts of N in the Gulf of Milazzo (February 2003) and in the Straits of Messina. These estimates suggested the highest potentiality of microbial assemblages inhabiting these two environments in decomposing organic polymers. The expression of high decomposition rates in these areas confirmed the importance of heterotrophic metabolism in oligotrophic Mediterranean ecosystems, in agreement with other studies [
9,
23,
35].
Moreover, because of the different substrate analogues used for measuring enzyme activity rates, the reciprocal ratios among the single ectoenzymatic activities (LAP/β-GLU, LAP/AP) were also calculated to better characterise and compare the different ecosystems under study (
Table 6).
Variations in the relative activities of different enzymes can also indicate geographical variations in the modalities of bacterial nutrition [
36]. High LAP/β-GLU ratios provide information on the flux of organic matter preferentially through proteins, while low ratios indicate a preferential flux of organic matter through polysaccharides. The highest values of this ratio were reached in the Gulf of Milazzo (February 2003), because of the lowest β-GLU levels; high values were also observed in the Gulf of Manfredonia and Milazzo (December 2002), two areas where similar low β-GLU activity rates were measured. High LAP/β-GLU values are generally found in many temperate coastal environments [
1,
18,
21,
23,
32], suggesting a higher potential degradation of proteins compared to that of polysaccharides. LAP values significantly (from 5 to 300 times) higher than β-GLU were also detected during recent investigations in the Adriatic Sea; on the other hand, the greater amount of LAP compared to β-GLU may be explained by the capability of released amino acids to provide a source of both C and N for bacteria [
18].
LAP/AP ratios provide insights on the relative importance of N mobilization compared to P mineralization. Sala
et al. [
37] suggested the use of AP/LAP ratio for evaluating P
versus N limitation of microbial communities “
in situ”. In our study, LAP/AP ratios were generally lower than 1, with a minimum value in the Gulf of Milazzo, during December 2002; this was the consequence of AP activity higher than LAP and suggested the greater importance of P mineralization. An exception was the Gulf of Milazzo during February 2003, when LAP prevailed over AP, so resulting in the highest LAP/AP ratio, and enhanced N mobilization.
In addition, in order to assess whether enzyme activities were mostly cell-associated, cell-specific activity rates were calculated through normalisation of the enzyme activities to the bacterioplankton abundance. For AP, which is synthesised by both phytoplankton and bacteria, cell-specific AP rates were also normalised to the concentration of Chl-
a, as a proxy of phytoplankton biomass. Scaling enzyme activity rates to the cell abundance allows to compare different environments for their degradation capability; nevertheless, this elaboration has some limitations, because a percentage of cells might be dormant or dead, different microbial groups could express different activity levels, and have intra-specific differences [
12,
17,
38]. Therefore, for this approach we assumed that all the microbial cells had similar activity levels. Specific LAP
per cell increased from the Northern Adriatic basin to the Gulfs of Manfredonia and Milazzo (December 2002); increases of about three times were found in the Straits of Messina, and values reached levels about four orders of magnitude higher in the Gulf of Milazzo (February 2003).
Cell-specific GLU ranged from the lowest activity values measured in the Gulf of Milazzo (February 2003) to the highest ones observed in the same area in December 2002 and particularly in the Straits of Messina; in these oligotrophic sites (
i.e., characterised by low Chl-
a,
Table 6), the presence of microorganisms with high potentiality in decomposing refractory material was confirmed. In the Southern Tyrrhenian Sea [
35], values of cell-specific LAP activity ranged from 6.5 to 23.1 amol/cell/h, while cell-specific β-GLU values ranged from 0.16 to 0.63 amol/cell/h (
i.e., corresponding to 0.47–1.66 fg C/cell/h and 0.011–0.45 fgC/cell/h for LAP and β-GLU, respectively).
Cell-specific AP values normalised to bacterioplankton abundance showed that the Gulf of Milazzo (December 2002) was the site with the most active bacterial community in processing organic phosphates. In addition, the Straits of Messina and the Gulf of Milazzo (February 2003) exhibited also high AP
per cell, normalised to bacterioplankton as well as to phytoplankton biomass. As the specific AP activity normalised to Chl-
a has been suggested to be a good indicator of P-limited algal growth [
39], this result indicated that phytoplankton reacted to this condition producing AP with higher specific activity. Cell specific AP values above 200 nmol/μg Chl-
a/h, corresponding to 6.20 μg P/μg Chl-
a/h, were obtained in areas of the central Atlantic Ocean with P-deficiency [
40].
Except for the Northern Adriatic Sea, cell-specific enzyme activities calculated in this study were higher than those reported in other coastal Mediterranean sites, such as in the Ligurian Sea [
23], where mean cell specific LAP and β-GLU were, on average, 1.8–3.96 fg C/cell/h and 0.072 fg C/cell/h, respectively, or in the NW Mediterranean (DYFAMED station) [
24], where mean cell specific AP was 0.062 fg P/cell/h. Cell-specific LAP and AP activity rates were particularly high in the Straits of Messina and in the Gulf of Milazzo, where the bacterioplankton abundances (mean value: 10
7 cells/l) were about two orders of magnitude lower than those found in the Northern Adriatic Sea. Therefore, high rates of cell-specific activity suggested the presence of a fraction of highly metabolically active bacteria able to release enzymes, rather than the whole bacterial community [
41].
Furthermore, it is important to note that, although only cell-associated enzyme activities have been assumed to be of ecological significance [
42–
44], the contribution of free, dissolved enzymes, to the total enzyme activity as another important source of enzymes in all the studied ecosystems cannot be excluded, as documented by other research performed in coastal [
21] as well as in oceanic [
45] areas. This could explain why significant relationships between enzyme activities and biotic components were not always detected.
As enzyme activity can be considered the initial response of the microbial community to environmental changes [
1,
21], attention was also paid to the environmental abiotic (physico- chemical) variables which were responsible for the observed variability in potential enzymatic activity patterns. In the Northern Adriatic Sea as well as in the Gulf of Manfredonia, salinity was the main environmental factor which affected enzyme patterns. Significant negative correlations between both LAP activity and salinity values, suggested the importance of fluvial input in providing allochthonous sources for microbial metabolism. The stratification of the “plume” outflow over the underlying waters was responsible for the local confinement of the trophic supply in the surface layer; this distribution was reflected by the vertical profiles of LAP and AP, showing a decreasing trend with increasing depth, similarly to what observed during previous studies [
14,
16]. The significant relationship of LAP and AP activity rates with POC found in the Northern Adriatic basin suggested that microorganisms responded to the available organic substrates stimulating specifically enzyme production.
In the Gulf of Manfredonia and in the Straits of Messina, the hydrological circulation patterns acted as the main factor regulating the enzyme distribution. In the Gulf of Manfredonia, Apulian coastal waters became enriched with organic matter coming from the Northern Adriatic basin, and further transported these organic inputs to the most southern transects; there, high AP and LAP activities were found within the first 50 m depth of the most off-shore stations. The coastal to offshore increasing trend followed by LAP activity rates supported the hypothesis that probably the particular circulation of water masses spread labile matter, not fully degraded in the coastal area, towards the offshore one. In the Straits of Messina, intense hydrodynamism such as that related to upwelling and cyclical changes of both Tyrrhenian and Ionian water masses, was supposed to modulate the nature and composition of organic inputs, determining, in turn, changes in the enzymatic spectra of microorganisms.
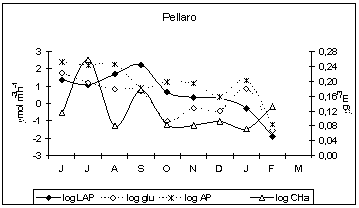
Changes in the water column structure (i.e., water stratification in late autumn and mixing in winter) were responsible for the temporal variations in the rates of organic matter processing recorded in the Gulf of Milazzo. When the water column was stratified, enhanced activity rates towards more refractory compounds, as shown by β-GLU values, were found. During water mixing, high LAP values suggested that the decomposition process was more active on newly produced material; this was also confirmed by the high Chl-a content.
Enzyme activity patterns reflected the diversity of biogeochemical features among the examined sites. In fact, we found distinct patterns of enzyme activities in each ecosystem and there was no consistent trend in the enzyme expression in relation to nutrient concentrations. In the Straits of Messina, the inverse relationship between LAP and nitrogen nutrients, observed during autumn at all the stations (LAP vs. NO3, r = −0.679, −0.749, −0.768, n = 12, P < 0.05 at Scaletta, Guardia and Pellaro, respectively), as well as during summer and winter at Scaletta station (LAP vs. NO3, r = −0.59, −0.627, n = 12, P < 0.05), led us to hypothesise a regulation of microbial attack on organic substrates by N rather than P. Also in the Gulf of Manfredonia, LAP was negatively related to NO3 (r = −0.259, n = 46, P < 0.05).
Concerning AP, the patterns observed in the present study indicated that the expression of AP was not always regulated by the concentration of its end-product; in fact, a significant inverse relation between AP and PO
4 concentration was observed in the Gulf of Manfredonia (r = −0.336, n = 46, P < 0.05) and in the Straits of Messina (r = −0.658, n = 40, P < 0.05, Pellaro station during autumn) only. In the Northern Adriatic basin, the lack of a significant inverse relation between AP and PO
4 (mean value 0.06 μM) could depend on the internal P stored inside the cells, as shown by Sebastian
et al. [
46]. Conversely, in the Gulf of Milazzo the synthesis of high cell-specific AP activity in spite of P availability (mean value: 2.99μM) could probably be a strategy adopted by the microbial community to get not only a Phosphorus but also a Carbon source, similarly to what reported by other Authors [
30,
38] at meso- and bathypelagic depths in oceanic and sub-tropical Atlantic waters.