Characterization of Streptomyces spp. Isolated from the Sea Surface Microlayer in the Trondheim Fjord, Norway
Abstract
:1. Introduction
2. Results and Discussion
2.1. A large proportion of cultivable neuston actinomycetes produce antimicrobial compounds
3. Experimental Section
3.1. Sampling and isolation of Streptomycete bacteria from sea surface microlayer (Sampling sites and sample collection)
3.2. Extraction and antimicrobial assay
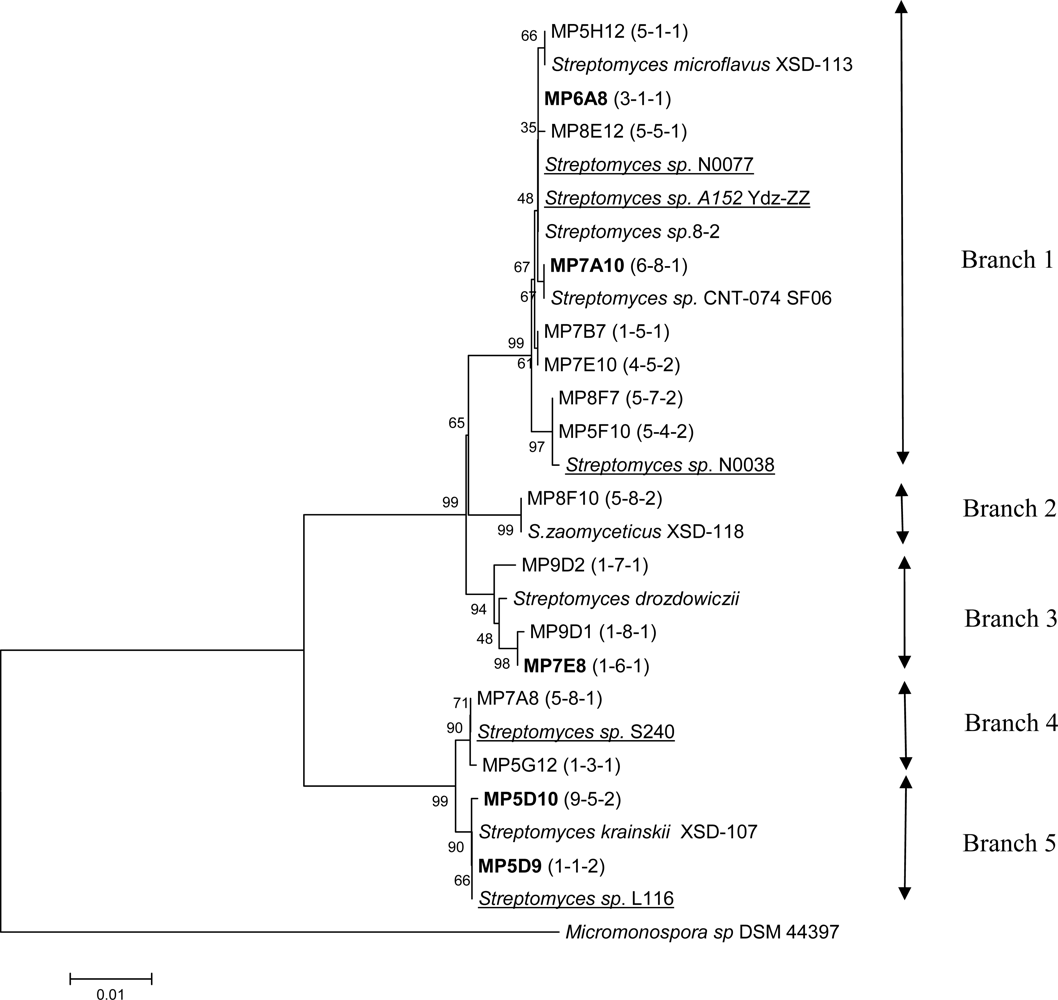
3.3 Cloning, sequencing and phylogenetic analysis
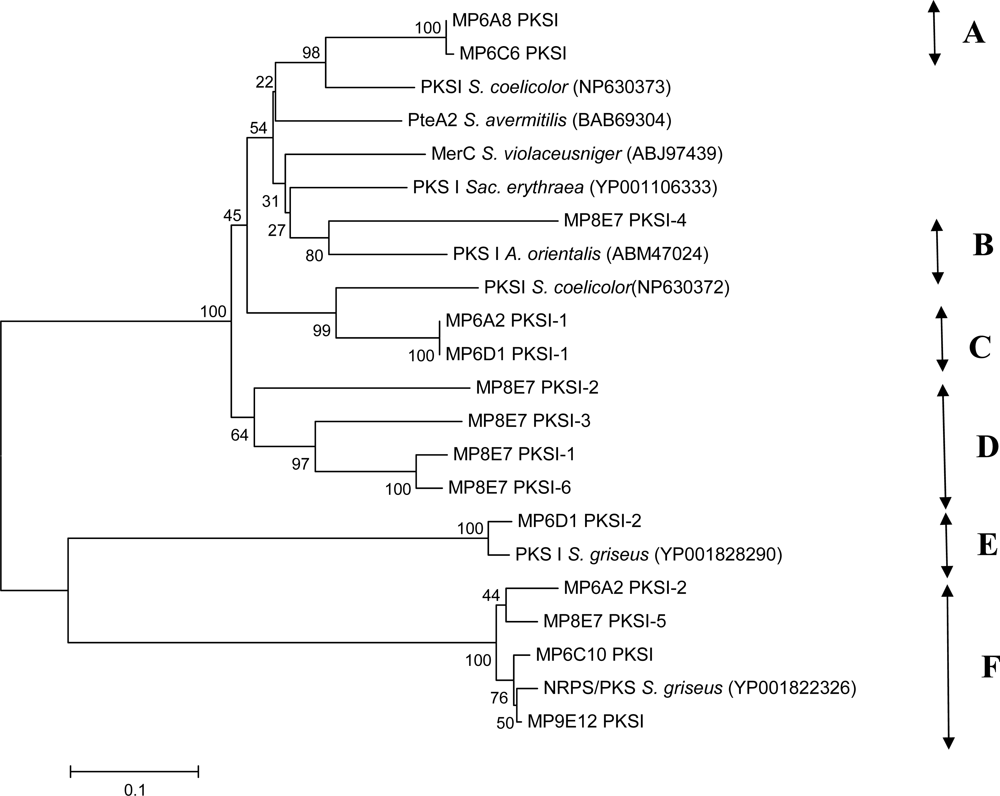
3.4 PCR amplification of PKS and NRPS-genes
3.5 Nucleotide sequence accession numbers
Acknowledgments
References and Notes
- Bull, AT; Stach, JEM. Marine actinobacteria: new opportunities for natural product search and discovery. TRENDS Microbiol 2007, 15, 491–499. [Google Scholar]
- Kelecom, A. Secondary metabolites from marine microorganisms. An. Acad. Bras. Ciênc 2002, 74, 151–170. [Google Scholar]
- Fiedler, H-P; Bruntner, C; Bull, AT; Goodfellow, AC; Potterat, O; Puder, C; Mihm, G. Marine actinomycetes as a source of novel secondary metabolites. Antonie van Leeuwenhoek 2005, 87, 37–42. [Google Scholar]
- Lemos, ML; Dopazo, CP; Toranzo, AE; Barja, JL. Competitive dominance of antibiotic-producing marine bacteria in mixed cultures. J Appl Bacteriol 1991, 71, 228–232. [Google Scholar]
- Wright, J. The production of antibiotics in soil. IV. Production of antibiotics in coats of seeds sown in soil. Ann. Appl. Biol 1956, 44, 561–566. [Google Scholar]
- Thomashow, LS; Weller, DM; Bonsall, RF; Oierson, LS. Production of the antibiotic phenazine-1-carboxylic acid by fluorescent Pseudomonas species in the rhizosphere of wheat. Appl. Environ. Microbiol 1990, 56, 908–912. [Google Scholar]
- Seymour, JR; Mitchell, JG; Pearson, L; Waters, RL. Heterogeneity in bacterioplankton abundance from 4.5 millimetre resolution sampling. Aquat. Microb. Ecolo 2000, 22, 143–153. [Google Scholar]
- Long, RA; Azam, F. Antagonistic interactions among marine pelagic bacteria. Appl. Environ. Microbiol 2001, 67, 4975–83. [Google Scholar]
- Hardy, JT; Word, JQ. Sea surface toxicity in Puget Sound. Puget Sound Notes. U.S. EPA Region 10: Seattle, WA, 1986; pp. 3–6, (As cited by GESAMP, 1995). [Google Scholar]
- Sieburth, J. Distribution and activity of oceanic bacteria. Deep-Sea Res. 1971, 18, 1111–1121, (as cited by GESAMP, 1995). [Google Scholar]
- Bezdek, HF; Carlucci, AF. Surface concentrations of marine bacteria. Limnol. Oceanogr 1972, 17, 566–569. [Google Scholar]
- Sieburth, J; Willis, PJ; Johnson, KM; Burney, CM; Lavoie, DM; Hinga, K R; Caron, DA; French, FW; Johnson, PW; Davis, PG. Dissolved organic matter and heterotrophic microneuston in the surface microlayers of the North Atlantic. Science 1976, 194, 1415–1418. [Google Scholar]
- Carlucci, AF; Craven, DB; Henrichs, SM. Surface-film microheterotrophs: amino acid metabolism and solar radiation effects on their activities. Mar. Biol 1985, 85, 13–22. [Google Scholar]
- Amann, RI; Ludwig, W; Schleifer, KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar]
- Agoguè, H; Casamayor, EO; Bourrain, M; Obernosterer, I; Joux, F; Herndl, GJ; Lebaron, P. 2005. A survey on bacteria inhabiting the sea surface microlayer of coastal ecosystems. FEMS Microbiol. Ecolo 2005, 54, 269–280. [Google Scholar]
- Jensen, PR; Gontang, E; Mafnas, C; Mincer, TJ; Fenical, W. Culturable marine actinomycete diversity from tropical Pacific Ocean sediments. Environ. Microbiol 2005, 7, 1039–1048. [Google Scholar]
- Peela, S; Kurada, VVSNB; Terli, R. Studies on antagonistic marine actinomycetes from the Bay of Bengal. World J. of Microbiol. & Biotechnol. 2005, 21, 583–585. [Google Scholar]
- Sponga, F; Cavaletti, L; Lazzarini, A; Borghi, A; Ciciliato, I; Losi, D; Marinelli, F. Biodiversity and potentials of marine-derived microorganisms. J. Biotechnol 1999, 70, 65–69. [Google Scholar]
- Zhao, H; Goodacre, R. Characterization of Streptomyces spp. isolated from coastal sediments. Unpublished. Submitted to GenBank 20.09.2004.
- Jensen, PR; Prieto-Davo, A; Fenical, W. Marine Derived Actinomycete Diversity. Unpublished. Submitted to GenBank 09.10.2007.
- Hong, K; Burgess, JG. Actinomycetes isolated from sediments collected in west coast of Scotland. Unpublished. Submitted to GenBank 24.04.2006.
- Zhang, H; Zhang, W; Jin, Y; Jin, M; Yu, X. A comparative study on the phylogenetic diversity of culturable actinobacteria isolated from five marine sponge species. Antonie Van Leeuwenhoek 2008, 93, 241–248. [Google Scholar]
- Hayakawa, Y; Sasaki, K; Adachi, H; Furihata, K; Nagai, K; Shinya, K. Thioviridamide, a novel apoptosis inducer in transformed cells from Streptomyces olivoviridis. J. Antibiotics 2006, 59, 1–5. [Google Scholar]
- Semêdo, LTAS; Gomes, RC; Linhares, AA; Duarte, GF; Nascimento, RP; Rosado, AS; Margis-Pinheiro, M; Margis, R; Silva, KRA; Alviano, CS; Manfio, GP; Soares, RMA; Linhares, LF; Coelho, RRR. Streptomyces drozdowiczii sp. nov., a novel cellulolytic streptomycete from soil in Brazil. Int. J. Syst. Evol. Microbiol 2004, 54, 1323–1328. [Google Scholar]
- Yoon, K-H; Lee, E-H; Kim, C-J; Yoon, K-H. Characterization and xylanase productivity of Streptomyces sp. WL-2. Han'gug mi'saengmul saengmyeong gong haghoeji 2005, 33, 178–183. [Google Scholar]
- Umezawa, H; Takeuchi, T; Aoyagi, T; Hamada, M; Ogawa, K; Iinuma, H. The novel physiologically active substance foroxymithine, a process and microorganisms for its production and its use as medicament. European Patent EP0162422.
- Schirmer, A; Gadkari, R; Reeves, CD; Ibrahim, F; DeLong, EF; Hutchinson, CR. Metagenomic analysis reveals diverse polyketide synthase gene clusters in microorganisms associated with the marine Sponge. Discodermia dissolute. Appl. Environ. Microbiol 2005, 71, 4840–4849. [Google Scholar]
- Busti, E; Monciardini, P; Cavaletti, L; Bamonte, R; Lazzarini, A; Sosio, M; Donadio, S. Antibiotic-producing ability by representatives of a newly discovered lineage of actinomycetes. Microbiol 2006, 152 Pt 3, 675–683. [Google Scholar]
- Wawrik, B; Kerkhof, L; Zylstra, GJ; Kukor, JJ. Identification of unique type II polyketide synthase genes in soil. Appl. Environ. Microbiol 2005, 71, 2232–2238. [Google Scholar]
- Bredholdt, H; Galatenko, OA; Engelhardt, K; Fjaervik, E; Terekhova, LP; Zotchev, SB. Rare actinomycete bacteria from the shallow water sediments of the Trondheim fjord, Norway: isolation, diversity and biological activity. Environ Microbiol 2007, 9, 2756–2764. [Google Scholar]
- Izumikawa, M; Murata, M; Tachibana, K; Ebizuka, Y; Fujii, I. Cloning of modular type I polyketide synthase genes from salinomycin producing strain of Streptomyces albus. Bioorganic & Medicinal Chem 2003, 11, 3401–3405. [Google Scholar]
- Sun, Y; Hong, H; Samboskyy, M; Mironenko, T; Leadlay, PF; Haydock, SF. Organization of the biosynthetic gene cluster in Streptomyces sp. DSM 4137 for the novel neuroprotectant polyketide meridamycin. Microbiol 2006, 152, 3507–3515. [Google Scholar]
- Oliynyk, M; Samborskyy, M; Lester, JB; Mironenko, T; Scott, N; Dickens, S; Haydock, SF; Leadlay, PF. Complete genome sequence of the erythromycin-producing bacterium Saccharopolyspora erythraea NRRL23338. Nat. Biotechnol 2007, 25, 447–453. [Google Scholar]
- Ikeda, H; Ishikawa, J; Hanamoto, A; Shinose, M; Kikuchi, H; Shiba, T; Sakaki, Y; Hattori, M; Omura, S. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol 2003, 21, 526–531. [Google Scholar]
- Banskota, AH; Mcalpine, JB; Sorensen, D; Ibrahim, A; Aouidate, M; Piraee, M; Alarco, AM; Farnet, CM; Zazopoulos, E. Genomic analyses lead to novel secondary metabolites. Part 3. ECO-0501, a novel antibacterial of a new class. J. Antibiot 2006, 59, 533–542. [Google Scholar]
- Pawlik, K; Kotowska, M; Chater, KF; Kuczek, K; Takano, E. A cryptic type I polyketide synthase (cpk) gene cluster in Streptomyces coelicolor A3(2). Arch. Microbiol 2007, 187, 87–99. [Google Scholar]
- Ohnishi, Y; Ishikawa, J; Hara, H; Suzuki, H; Ikenoya, M; Ikeda, H; Yamashita, A; Hattori, M; Horinouchi, S. Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J. Bacteriol 2008, 190, 4050–4060. [Google Scholar]
- Moffitt, MC; Neilan, BA. Evolutionary affiliations within the superfamily of ketosynthases reflect complex pathway associations. J. Mol. Evol. 2003, 56, 446–457. [Google Scholar]
- Kjelleberg, S; Stenström, TA; Odham, G. Comparative study of different hydrophobic devices for sampling lipid surface films and adherent microorganisms. Mar. Biol 1979, 53, 21–25. [Google Scholar]
- Atlas, RM. Handbook of Microbiological Media, 2nd Ed ed; CRC Press, Inc: New York, NY, 1997; p. 54. [Google Scholar]
- Burkholder, P; Pfister, R; Leitz, F. Production of a pyrrole antibiotic by a marine bacterium. Appl. Microbiol 1966, 14, 649–653. [Google Scholar]
- Lane, DJ. 16S/23S rRNA Sequencing. In Nucleic acid Techniques in Bacterial Systematics; Stackebrandt, E, Goodfellow, M, Eds.; John Wiley & Sons: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
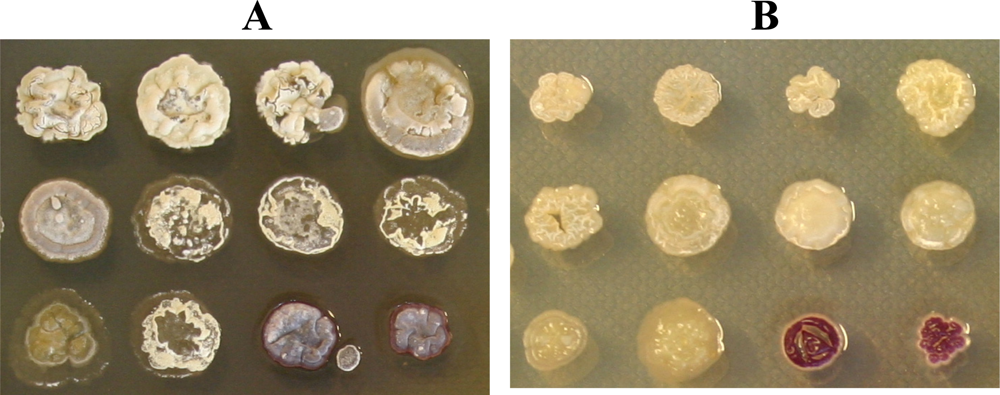
| Group | Characteristics | ||
|---|---|---|---|
| SM | AM | Other | |
| 1 | Colourless | White | |
| 2 | Colourless | White | Produces yellow metabolite diffusing in solid media |
| 3 | Colourless | Greenish-white | |
| 4 | Colourless | Greenish-white | Produces yellow metabolite diffusing in solid media |
| 5 | Light brown | Light grey | |
| 6 | Brown /greenish | Grey | |
| 7 | Colourless /light brown | Light purple | |
| 8 | Red | None | |
| 9 | Red | White | |
| 10 | Yellow | None | Flaky |
| Group (G1-10) and sample number (S1, S2) | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibition | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 | |||||||||||||||
| Nr | C | M | E | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | Total | |
| 1 | x | x | x | 6 | 7 | 0 | 1 | 5 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 2 | 0 | 0 | 29 | |
| 2 | x | x | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | ||
| 3 | x | x | 9 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | ||
| 4 | x | 28 | 14 | 4 | 0 | 0 | 2 | 0 | 0 | 4 | 3 | 3 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 62 | |||
| 5 | x | x | 4 | 7 | 1 | 3 | 2 | 2 | 7 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 8 | 3 | 1 | 1 | 0 | 45 | ||
| 6 | x | 5 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 11 | |||
| 7 | x | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | |||
| 8 | 12 | 7 | 2 | 0 | 1 | 0 | 0 | 0 | 5 | 4 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | ||||
| Sum, no of isolates | 72 | 42 | 7 | 4 | 8 | 5 | 13 | 2 | 13 | 8 | 11 | 6 | 2 | 0 | 3 | 13 | 3 | 3 | 2 | 0 | 217 | ||||
| Sum, no of isolates | 114 | 11 | 13 | 15 | 21 | 17 | 2 | 16 | 6 | 2 | |||||||||||||||
| Antifungal (%) | 66 | 45 | 54 | 13 | 43 | 41 | 0 | 38 | 33 | 0 | |||||||||||||||
| Antibacterial (%) | 46 | 45 | 77 | 1 00 | 24 | 12 | 100 | 94 | 100 | 100 | |||||||||||||||
| No activity (%) | 17 | 18 | 8 | 0 | 43 | 53 | 0 | 0 | 0 | 0 | |||||||||||||||
| Isolate | Morphology group | Sample number | Inhibition | ||
|---|---|---|---|---|---|
| C | M | E | |||
| MP6A2 | G4 | S1 | x | x | |
| MP6A8 | G3 | S1 | x | x | x |
| MP6C6 | G4 | S1 | x | ||
| MP6C10 | G1 | S1 | x | x | |
| MP6D1 | G2 | S1 | x | ||
| MP8E7 | G10 | S1 | x | ||
| MP9E12 | G1 | S2 | |||
Share and Cite
Hakvåg, S.; Fjærvik, E.; Josefsen, K.D.; Ian, E.; Ellingsen, T.E.; Zotchev, S.B. Characterization of Streptomyces spp. Isolated from the Sea Surface Microlayer in the Trondheim Fjord, Norway. Mar. Drugs 2008, 6, 620-635. https://doi.org/10.3390/md6040620
Hakvåg S, Fjærvik E, Josefsen KD, Ian E, Ellingsen TE, Zotchev SB. Characterization of Streptomyces spp. Isolated from the Sea Surface Microlayer in the Trondheim Fjord, Norway. Marine Drugs. 2008; 6(4):620-635. https://doi.org/10.3390/md6040620
Chicago/Turabian StyleHakvåg, Sigrid, Espen Fjærvik, Kjell D. Josefsen, Elena Ian, Trond E. Ellingsen, and Sergey B. Zotchev. 2008. "Characterization of Streptomyces spp. Isolated from the Sea Surface Microlayer in the Trondheim Fjord, Norway" Marine Drugs 6, no. 4: 620-635. https://doi.org/10.3390/md6040620




