1. Introduction
Sponges belonging to the
Cliona genus have proven to be a rich source of metabolites from derived from aminoacids. Some representative examples are clionamide [
1] and celenamides A-D [
2] isolated from
C. celata and stornamides A-D [
3] from an unidentified species of this genus, presenting some of them antibacterial activities.
As a result of our studies on the more polar fractions of the methanolic extracts from the sponge
Cliona celata collected at the Galicia Coast (NW Spain), in 1996 we reported the isolation and the spectroscopic elucidation of the aminoacid derivative acetylhomoagmatine (
1) which structure is characterized by the presence of a guanidine group [
4].
Due to the important function played for some polyamines bearing guanidine moieties in growth regulation and cancer biology, e.g. 1-guanidino-7-aminoheptane suppressed tumor cell growth by inhibition of deoxyhypusine synthase, the structure of acetylhomoagmatine can be a good target to study this kind of activity [
5]. On the other hand, and taking in account the lethal coral activity shown by siphonidictidine, another guanidine containing metabolite isolated from
Siphonodictyon sp., against
Acropora formosa coral gave rise the indication of the other possible role of this compound in the marine organism [
6]. For all these reasons, in this paper we wish to report the first total synthesis of acetylhomoagmatine in order to perform the biological studies related to the former activities.
2. Results and Discussion
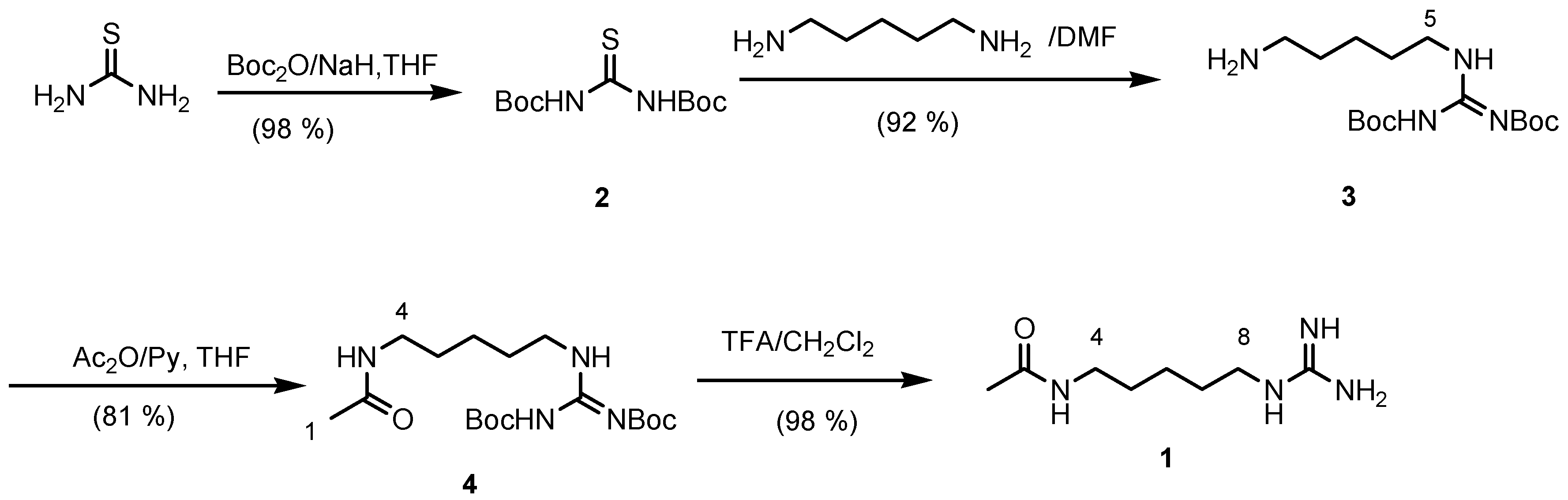
The synthesis begun from thiourea which was treated with sodium hydride and an excess of di-
tert-butyldicarbonate to give the protected
N,N-di-
tert-butoxycarbonyl thiourea (
2). Reaction of
2 with cadaverine afforded the required protected aminoguanidine homoagmatine (
3) in a 92 % yield. Acetylation of compound
3 with acetic anhydride in pyridine followed by deprotection by treatment with TFA in dichloromethane gave
1 in a quantitative yield (
Figure 1). The spectral data of the synthetic product are identical with those of natural product.
The synthetic acetylhomoagmatine was studied in vitro on A-549, HCT-116, PSN1, and T98G tumor cells, showing a very mild cytotoxic and selective activity (IC50 10 μg/mL) against A-549. The studies of allelophatic activity are underway.
3. Experimental Section
General Experimental Procedures. The 1H and 13C NMR spectra were recorded on a Bruker AVANCE 300 spectrometer using CDCl3 as solvent unless otherwise stated. EIMS and FABMS were recorded on a VG-Quattro instrument. HRESI-MS was performed in APEX III FT/MS equipped with a ESI probe. All reactions were run under an atmosphere of argon. THF was distilled from sodium benzophenone ketyl. DMF was store in a bottle with 4Å molecular sieves. CH2Cl2 and pyridine were distilled from CaH2 and KOH respectively.
Inhibition of Cell Growth by Cell Counting. This form of assay employs 96-well microplates. The tumor cell lines employed are: Human lung carcinoma A549 (ATCC CCL-185), colon adenocarcinoma HCT-116 (ATCC CCL-247), Human Caucasian glioblastoma multiforme T98G (ECACC 92090213) and human pancreatic adenocarcinoma PSN1 (ECACC 94060601). These were cultured in RPMI medium containing glutamine (2 mM), penicillin (50 IU/mL), streptomycin (50 μg/mL), supplemented with 5% FBS (A549 and HCT-116) or 10% FBS (PSN1), T98G was maintained in RPMI 1640, 2 mM glutamine, penicillin (50 IU/mL), streptomycin (50μg/mL), 1 mM Pyruvate supplemented with amino acids and 10% FBS.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT; Sigma Chemical Co., St. Louis, MO) dye reduction assay in 96-well microplates was used, essentially as described [
7]. The assay is dependent on the reduction of MTT by mitochondrial dehydrogenases of viable cells to a blue formazan product, which can be measured spectrophotometrically. For each experiment the cells were harvested from subconfluent cultures using trypsin and resuspended in fresh medium before planting. The tumor cells were incubated in each well with serial dilutions of the tested compounds in 200 μL of complete medium. A separate set of wells was seeded as a growth control to ensure that cells remained in the exponential growth phase. After 2 days of incubation at 37 °C and 5% CO
2 in an atmosphere with 98% humidity, MTT (5mg/ml in PBS) were added to each well and the plate was incubated for a further 2 h (37 ºC). The resulting formazan was dissolved in DMSO and read at 490 nm. All determinations were carried out in triplicate. The IC
50 value was calculated as the concentration of drug yielding 50% cell survival by comparing the OD in wells with drug to the OD in the control wells.
N, N-di-tert-butoxycarbonyl thiourea (2)
The compound
2 was prepared by the method reported in the literature [
7] as a white powder in a 98% yield.
Di-Boc-homoagmatine (3)
The compound
3 was prepared by the method reported in the literature [
8] as colorless oil in a 92% yield.
Acetyl di-Boc-homoagmatine (4)
A solution of 3 (0.112 g, 0.36 mmol) and 80 μL of dry pyridine in 2.0 mL of dry THF was cooled to 0 ºC and then, 47 μL of acetic anhydride (0.43 mmol) was added dropwise during 30 min. The mixture was stirred for 10 min, and after room temperature was reached, the solution was allowed to stand for 1 h. The reaction mixture was extracted with EtOAc (5 × 25 mL) and CH2Cl2 (5 × 25 mL), and the organic layers washed with brine and dried over anhydrous Na2SO4. After solvent evaporation 0.104 g of 4 was obtained (0.29 mmol, 81 %). 1H NMR (300 MHz, CDCl3) δ (ppm): 3.41 (H-8, q, J=6.8, 2H); 3.24 (H-4, q, J= 6.9, 2H); 1.98 (H-1, 3H); 1.53–1.61 (m, 2H); 1.48 (CH3, bs. 18H); 1.36–1.48 (m, 4H). 13C NMR (75 MHz, CDCl3) δ (ppm): 170.08 (C-2, s); 163.56 (C-10, s); 156,16 (C=O, s); 153.32 (C=O, s); 83.11 (C-O, s); 79.28 (C-O, s); 40.62 (C-4, t); 39.34 (C-8, t); 29.68 (C-5, t); 29.12 (C-7, t); 24.05 (C-6, t); 28.28 (CH3, q); 28.06 (CH3, q); 23.30 (C-1, q). EIMS, m/z (relative intensity): C18H34N4O5 386 [M]+ (9); 314 [M-CH3CONHCH2]+ (11); 300 [M-CH3CONH( CH2)2]+ (1); 286 [M-CH3CONH(CH2)3]+ (3); 272 [M-CH3CONH(CH2)4]+ (4); 258 [M-CH3CONH( CH2)5]+ (5); 126 (47);100 [C5H10NO]+ (69); 86 [C4H8NO]+ (100).
Acetylhomoagmatine (1)
A solution of 4 (0.170 g, 0.44 mmol) in 5 mL of dry CH2Cl2 was cooled to 0 ºC and then, 3 mL of TFA was added dropwise during 15 min. The mixture was stirred for 1h, until room temperature was achieved. The final solution was left for 3 h, and after solvent was evaporated, 80 mg of 1 was obtained as colorless oil (0.43 mmol, 98 %). 1H NMR (300 MHz, CD3OD) δ (ppm): 3.16 (H-4 y H-8, br t, J = 9.5 Hz, 4H); 1.93 (H-1, s, 3H); 1.59 (H-5, m, 2H); 1.52 (H-7, m, 2H); 1.39 (H-6, m, 2H). 13C NMR (75 MHz, CD3OD) δ (ppm): 173.34 (C=O, s); 158.59 (C=N, s); 42.22 (C-4, t); 40.12 (C-8, t); 29.80 & 29.36 (C-5/C-7, t); 24.84 (C-6, t); 22.53 (C-1, q). (+) LRFABMS m/z, (%): 209 [M + Na] + (8), 187 [M + H]+ (100). (+) HRESIMS m/z, (%) 187.1555 [M + H]+ (calcd for C8H19N4O, 187.1553).