Identification and Biological Characterization of Angiogenic and Tumor Growth Inhibitors derived from Sinica cetorhinus maximum Cartilage
Abstract
:Introduction
Results and Discussion
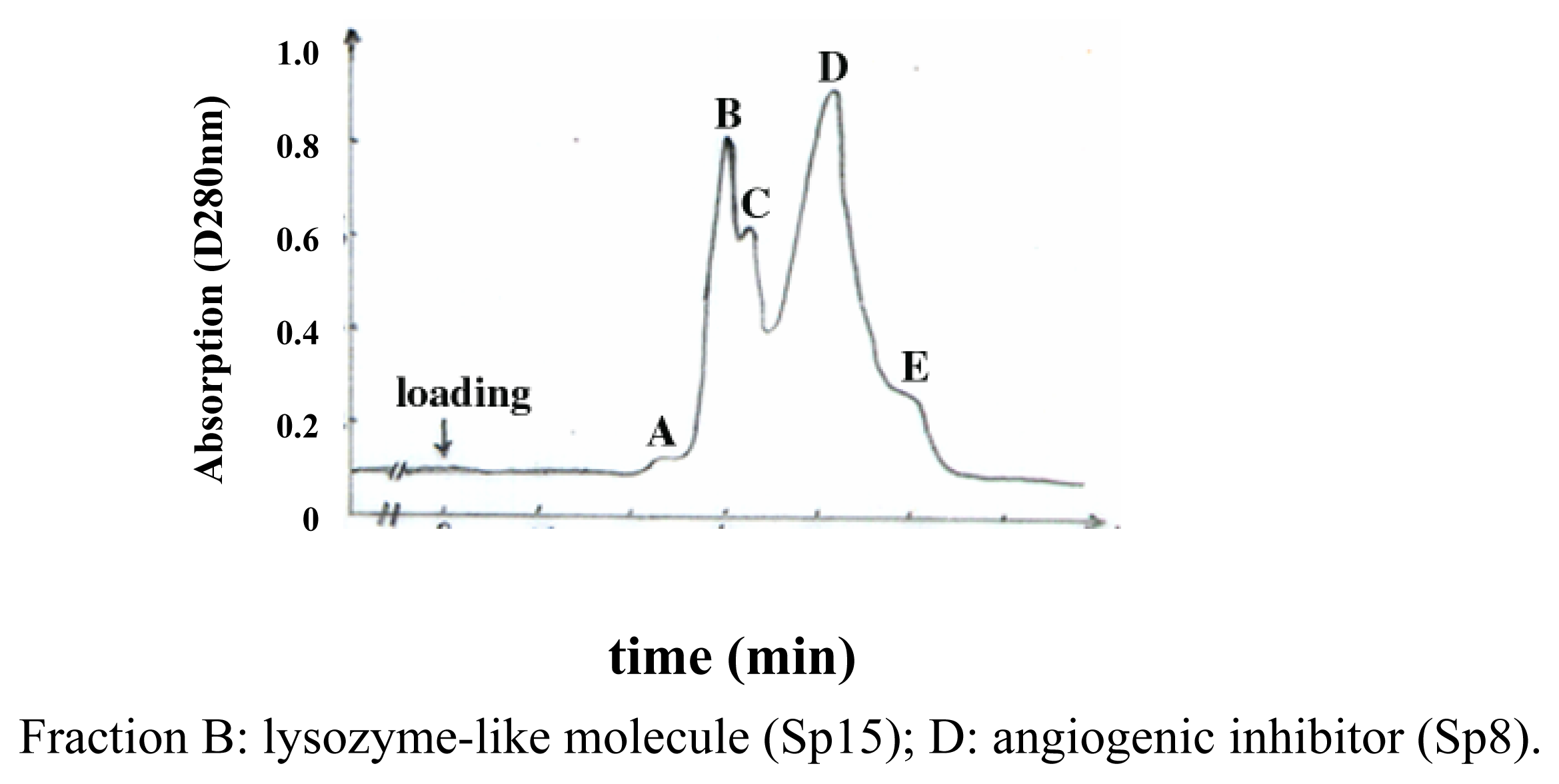
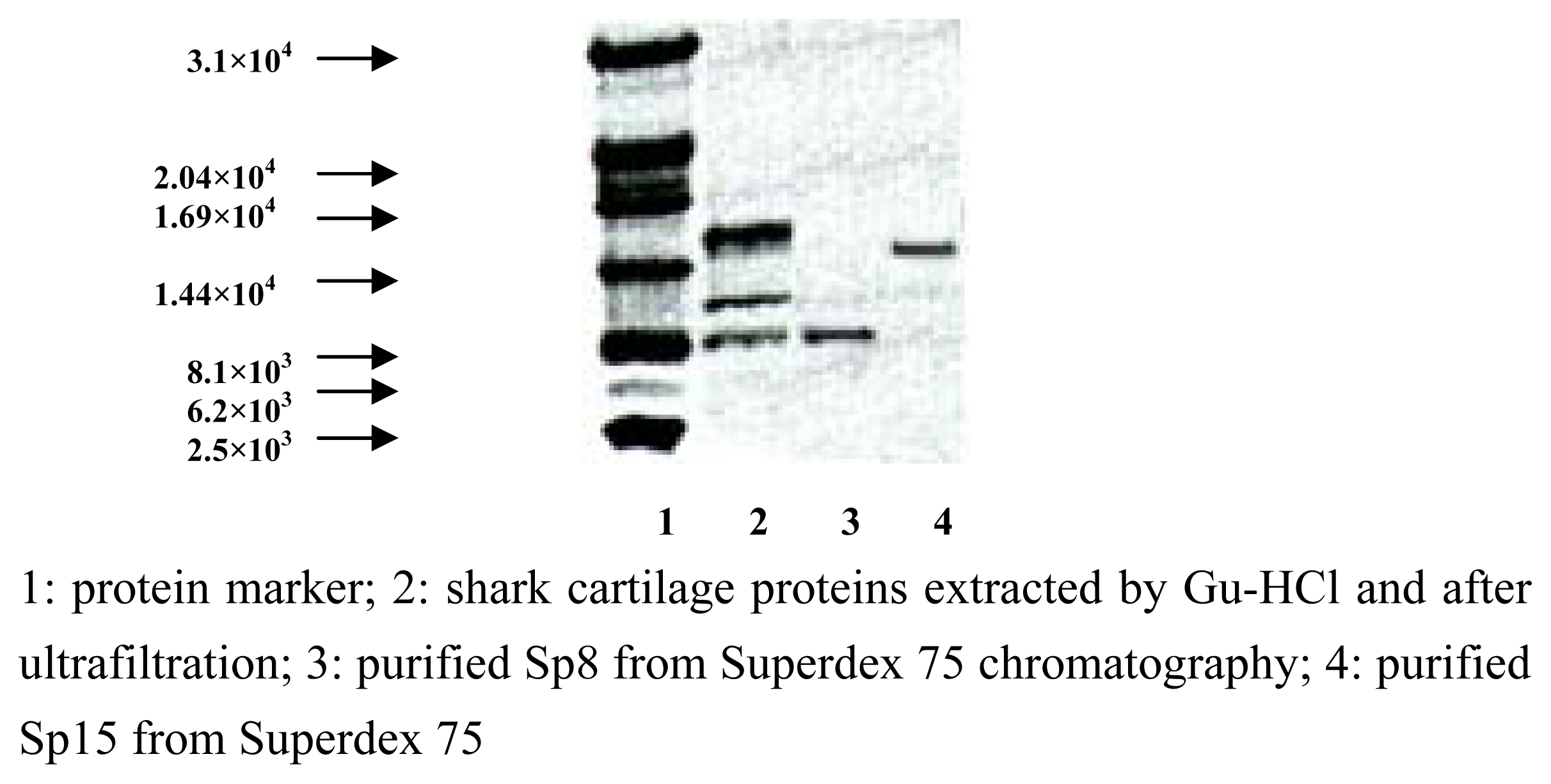
Purification and identification of Sp15 and Sp8
Determination of the N-terminus and lysozymatic activity of Sp15
Inhibition of endothelial cell proliferation
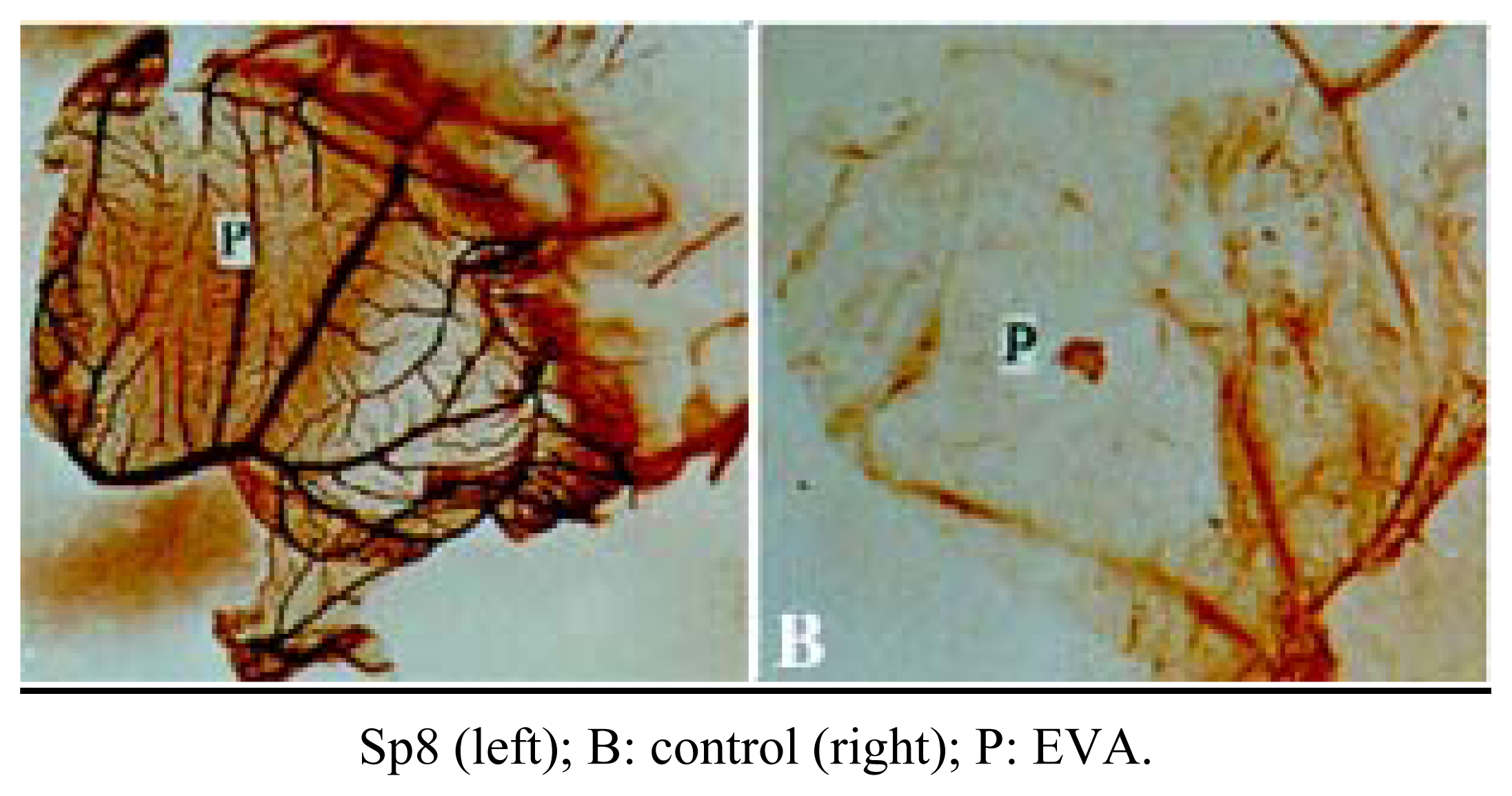
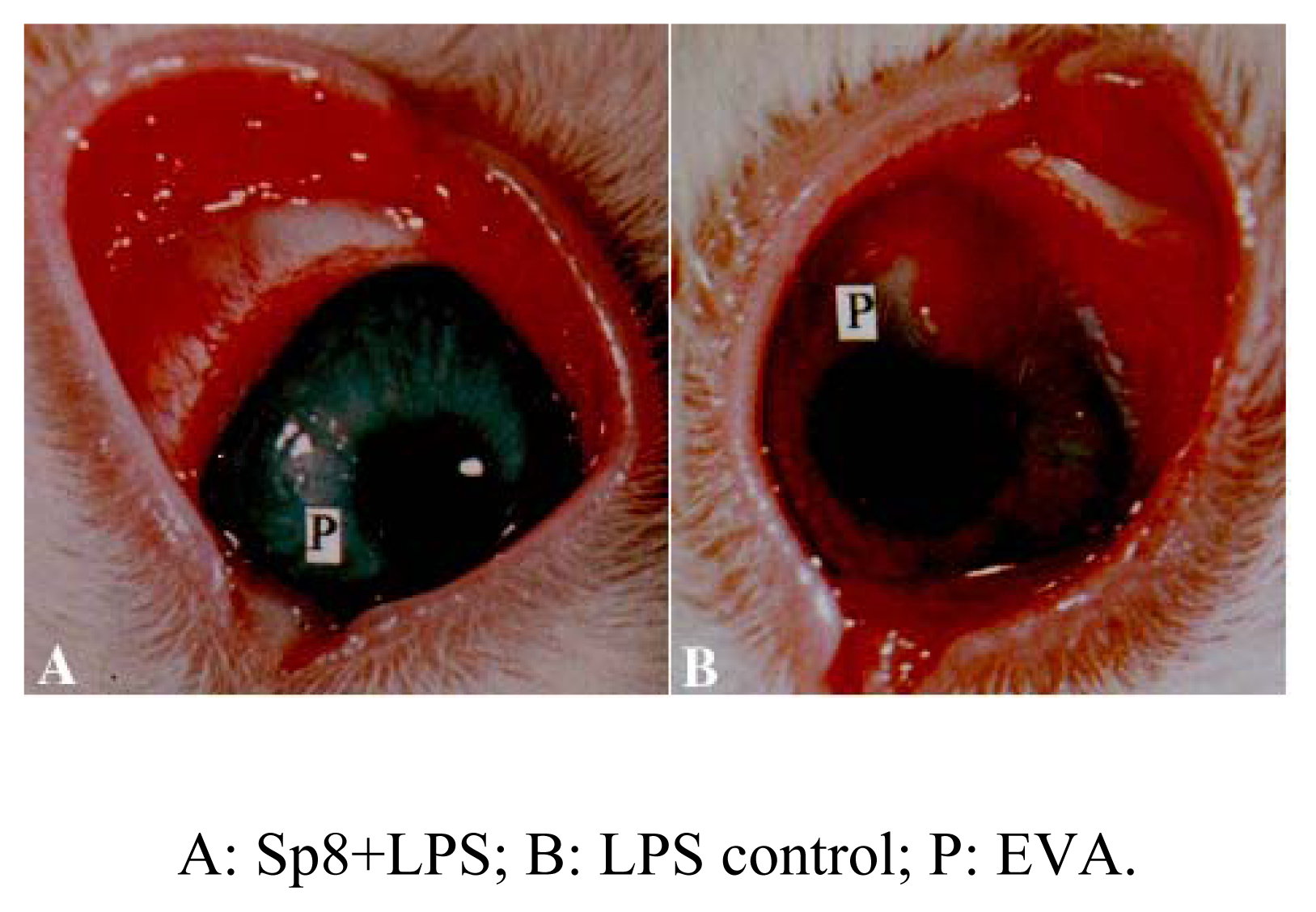
Inhibition of angiogenesis of Sp8 in vivo
Anti-tumor activity of Sp15 and Sp8 in vivo
Conclusions
Experimental
General
Extraction and Purification
Analytical
| Sample a | Angiogenesis | Inhibition rate (%) c | ||
|---|---|---|---|---|
| Length (mm) | Clock No. | Area (mm2) b | ||
| LPS | 5.0±0.3 | 5.6 | 46.90±4.32 | 0 |
| Sp8+LPS | 1.2±0.2 | 4.0 | 12.82±3.13 | 72.7** |
| Sample | Dosage (mg/kg) | Weight of tumor (g±x) | Inhibitory rateb (%) |
|---|---|---|---|
| Sp15 | 2.0 | 0.90±0.09 | 0 |
| 4.0 | 0.69±0.15 | 23.3* | |
| 8.0 | 0.60±0.11 | 33.3** | |
| Elyc | 20.0 | 0.81±0.18 | 10.0* |
| Sp8 | 0.050 | 0.71±0.12 | 21.1* |
| 0.100 | 0.41±0.11 | 54.4** | |
| 0.200 | 0.39±0.05 | 56.7** | |
| CTXd | 3.0 | 0.62±0.13 | 31.0** |
| PBS | - | 0.90±0.15 | - |
- Samples Availability: Available from the authors.
References and Notes
- Hahnfeldt, P.; Panigrahy, D.; Folkman, J.; Hlatky, L. Tumor development under angiogenic signaling: dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res 1999, 59, 4770–4775. [Google Scholar]
- Ferrara, N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 2002, 29 6 Suppl 16, 10–4. [Google Scholar]
- Wang, L.; Jiao, B.; Miao, H. Isolation, purification and physico-chemical characterization of an angiogenetic inhibitor derived from Sinica cetorhinus maximum cartilage. Pharm Biotech 1997, 4, 17–21. [Google Scholar]
- Langer, R.; Brem, H.; Falterman, K.; Klein, M.; Folkman, J. Isolation of a cartilage factor inhibits tumor neovascularization. Science 1976, 193, 70–72. [Google Scholar]
- Oikawa, T.; Ashino-Fuse, H.; Shimamura, M.; Koide, U; Lwaguchi, T. A novel angiogenic inhibitor derived from Japanese shark cartilage (I): Extraction and estimation of inhibitory activities toward tumor and embryonic angiogenesis. Cancer Lett 1990, 51, 181–186. [Google Scholar]
- Liang, J.H; Wong, K.P. The characterization of angiogenesis inhibitor from shark cartilage. Adv Exp Med Biol 2000, 476, 209–223. [Google Scholar]
- Brem, H.; Folkman, J. Inhibition of tumor angiogenesis mediated by cartilage. J Exp Med 1975, 141, 427–439. [Google Scholar]
- Dupont, E.; Brazeau, P.; Juneau, C. Extracts of shark cartilage having an antiangiogenic activity and an effect on tumor regression; process of making thereof. United States Patent 5618925, 1997. [Google Scholar]
- Boivin, D.; Gendron, S.; Beaulieu, E.; Gingras, D.; Beliveau, R. The antiangiogenic agent Neovastat (AE-941) induces endothelial cell apoptosis. Mol Cancer Ther 2002, 1, 795–802. [Google Scholar]
- Beliveau, R.; Gingras, D.; Kruger, E.; Lamy, S.; Sirois, P.; Simard, B.; Sirois, M.G.; Tranqui, L.; Baffert, F.; Beaulieu, E.; Dimitriadou, V.; Pepin, M.-C.; Courjal, F.; Ricard, I.; Poyet, P.; Falardeau, P.; Figg, W.D; Dupont, E. The antiangiogenic agent neovastat (AE-941) inhibits vascular endothelial growth factor-mediated biological effects. Clin Cancer Res 2002, 8, 1242–1250. [Google Scholar]
- Sava, G.; Ceschia, V.; Pacor, S. Mechanism of the antineoplastic action of lysozyme: evidence for host mediated effects. Anticancer Res 1989, 9, 1175–1180. [Google Scholar]
- Chen, S.F.; Fei, X.; Li, S.H. A new simple method for the isolation of microvascular endothelial cells. Microvasc. Res 1995, 50, 119–128. [Google Scholar]
- DeClerck, Y.A.; Laug, W.E. Inhibition of tumor cell collagenolytic activity bovine endothelial cells. Cancer Res 1986, 46, 3580–3586. [Google Scholar]
- Schagger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range 1 to 100 KDa. Anal. Biochem 1987, 166, 368–379. [Google Scholar]
- Langer, R.; Folkman, J. Polymer for sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [Google Scholar]
- Taylor, S.; Folkman, J. Protamine is an inhibitor of angiogenesis. Nature 1982, 297, 307–312. [Google Scholar]
© 2004 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Jiao, B.; Chen, J.; Miao, W.; Wang, L.; Zhu, Y.; Miao, H. Identification and Biological Characterization of Angiogenic and Tumor Growth Inhibitors derived from Sinica cetorhinus maximum Cartilage. Mar. Drugs 2004, 2, 30-38. https://doi.org/10.3390/md201030
Jiao B, Chen J, Miao W, Wang L, Zhu Y, Miao H. Identification and Biological Characterization of Angiogenic and Tumor Growth Inhibitors derived from Sinica cetorhinus maximum Cartilage. Marine Drugs. 2004; 2(1):30-38. https://doi.org/10.3390/md201030
Chicago/Turabian StyleJiao, Binghua, Jianhe Chen, Weimin Miao, Lianghua Wang, Yuping Zhu, and Huinan Miao. 2004. "Identification and Biological Characterization of Angiogenic and Tumor Growth Inhibitors derived from Sinica cetorhinus maximum Cartilage" Marine Drugs 2, no. 1: 30-38. https://doi.org/10.3390/md201030




