New Marine Derived Anticancer Therapeutics ─ A Journey from the Sea to Clinical Trials
Abstract
:“Nature herself must be our advisor; the path she chalks must be our walk. For as long as we confer with our own eyes, and make our ascent from lesser things to higher, we shall be at length received into her closest-secrets.”William HarveyDe Generatione Animalium, 1651.
Introduction
Historical Perspective and Current Status
Marine Derived Anticancer Agents
ET-743 (Trabectedin, Yondelis™)
AplidinR (APL)
Kahalalide F (KF)
Development of Marine Derived Therapeutics
The Challenge
Conclusions
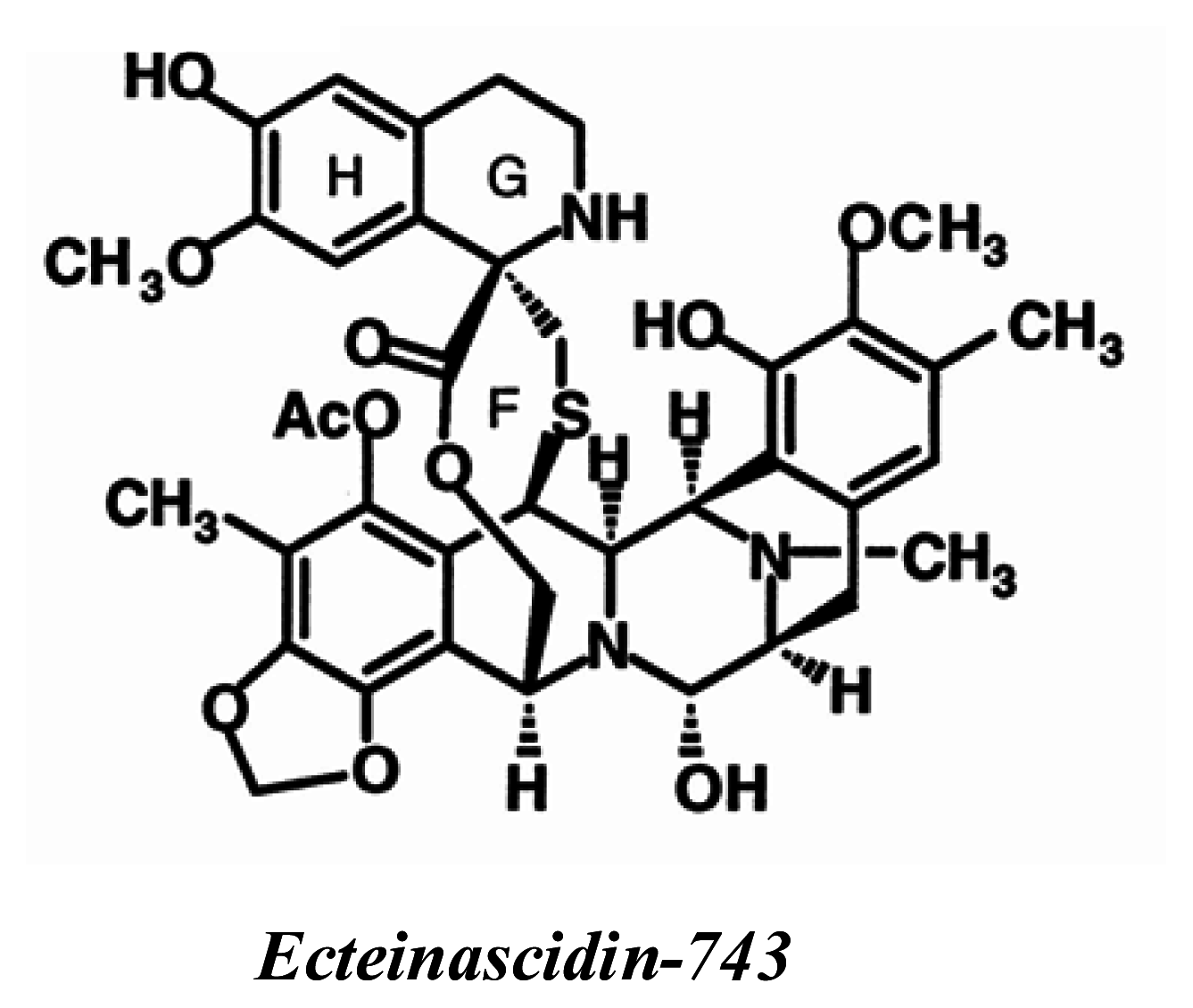
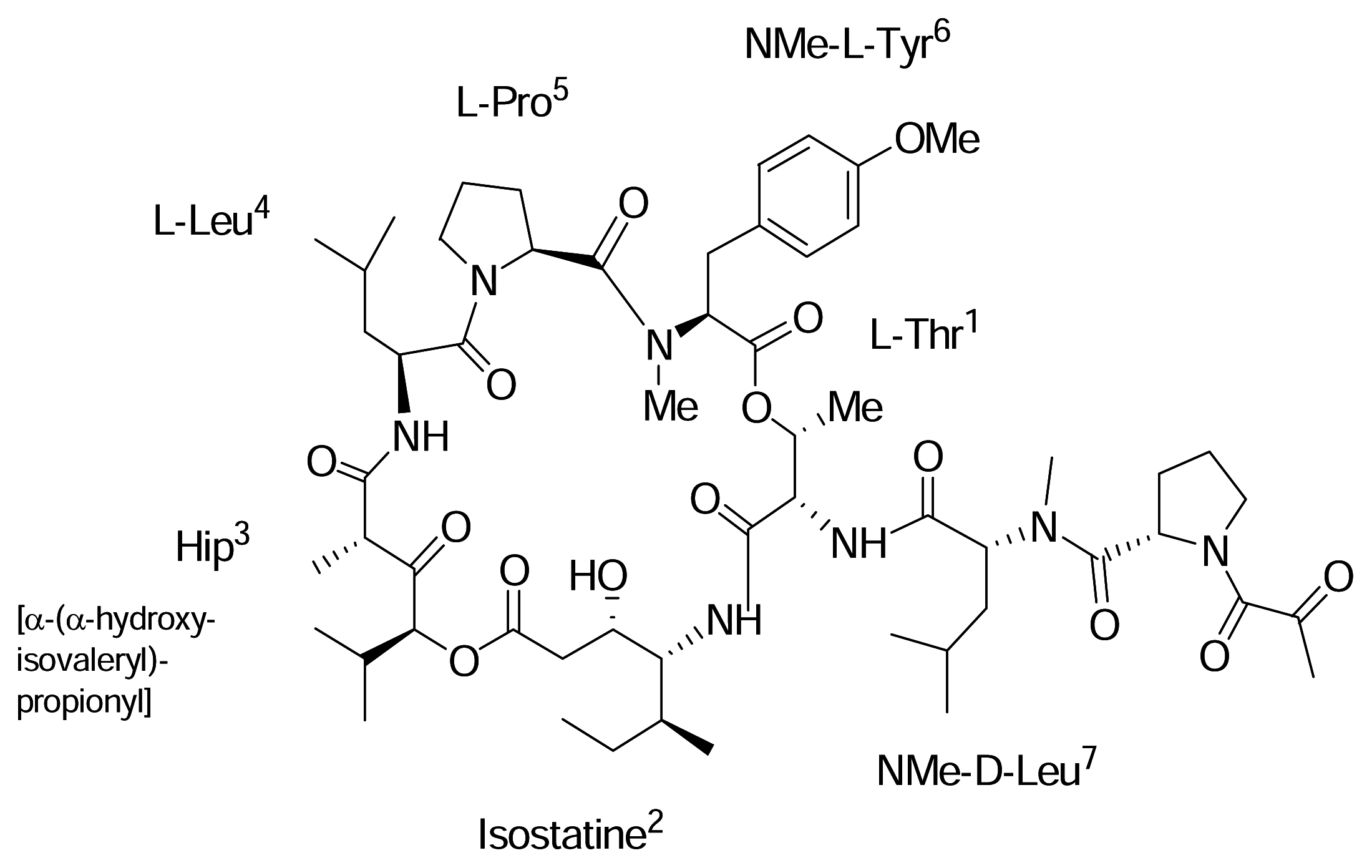
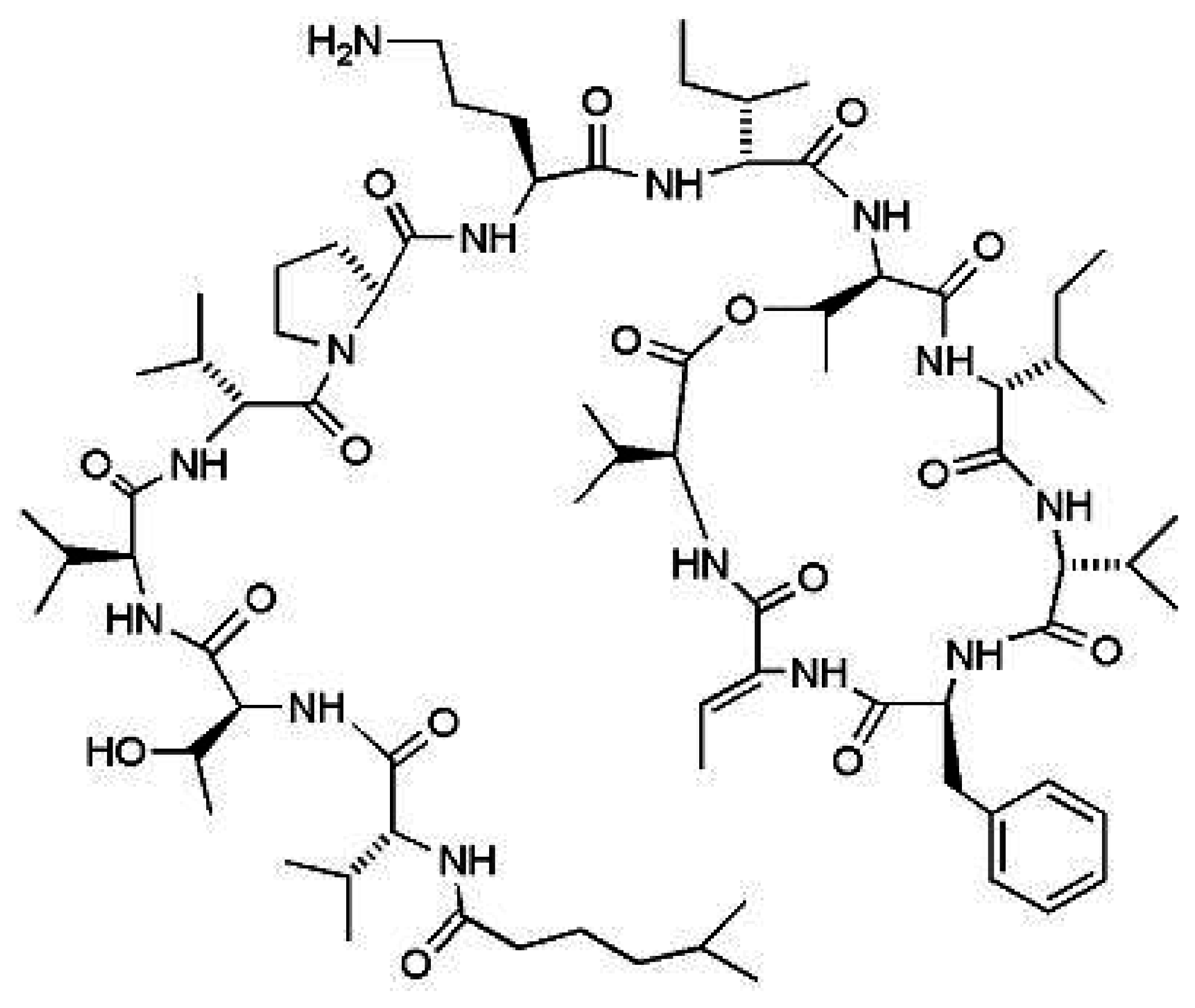
|
|
| COMPOUND | SOURCE | CHEMICAL CLASS | PROPOSED CELLULAR TARGET(S) |
|---|---|---|---|
| Yondelis | Tunicate | Isoquinolone |
|
| Aplidin | Tunicate | Cyclic Peptide |
|
| Kahalalide F | Green Algae | Cyclic Peptide |
|
- †“ This paper is dedicated to the memory of P J. Scheuer (1916–2003) a pioneer in marine drug research and discoverer of Kahalalide F and to the 2.184 cancer patients that have given their informed consent to participate in our marine derived clinical anticancer program”.
References
- Monnier, C. Les plantes Medicinales. Vertus et Traditions; Privat Eds. Toulouse, 2002. [Google Scholar]
- Sweldlow, J. Nature and Medicine; National Geographic Mil Eds.: Paris, 2000. [Google Scholar]
- Ellis, R. Aquagenesis. The origin and evolution of life in the sea; Viking Eds.: New York, 2001. [Google Scholar]
- Macdougall, J.D. A short history of Planet Earth; John Wiley Eds.: New York, 1996; p. 5. [Google Scholar]
- Argulis, L.; Schwartz, K. Five Kingdoms, an illustrated guide to the phyla of life on Earth; W.H. Freeman & Company: New York, 1982; pp. 16–17. [Google Scholar]
- Scotfield, V.I.; Schumpberger, J.M.; West, L.A.; Weissman, L. Protocordate allorecognition is controlled by aq MHC-like gene. Nature 1982, 295, 499–502. [Google Scholar]
- Pendergast, R.A.; Lutty, G.A.; Scott, L. Direct inflammation: the phylogeny of lymphokines. Dev. Comp. Inmunol 1983, 7, 629–632. [Google Scholar]
- Ruggieri, G.D. Drugs from the sea. Science 1976, 194, 491–496. [Google Scholar]
- Haefner, B. Drugs from the deep. Marine natural products as drug candidates. Drug Discov Today 2003, 8, 536–544. [Google Scholar]
- Sorriente, A. Manoalide. Current Med Chemistry 1999, 6, 415–431. [Google Scholar]
- Longley, R.; Cardigan, D.; Harmody, D.; Gunasereka, M.; Gunasereka, S. Discodermolide - A new marine derived Inmunosuppressive compound. Transplantation 1991, 52, 650–661. [Google Scholar]
- Angerhofer, C.; Pezzuto, J.; Konig, G.; Wright, A.; Sticcher, O. Antimalarial activity of Sestiperquenes from the marine sponge Acanthella klelthra. J. Nat. Prod 1992, 55, 1787–1789. [Google Scholar]
- Nagai, H.; Murata, M.; Torigoe, K.; Sakate, M.; Yasumoto, T. Gambieric acids, New potent antifungal substances with unprecedent polyether structures from a marine dinoflagelate Gambiercus toxicus. J. Org. Chem 1992, 57, 5448–5453. [Google Scholar]
- Alonso, D.; Khalil, Z.; Satkunanthan, N.; Livett, B.G. Drugs from the Sea: Conotoxins as Drug Leads for Neuropathic Pain and Other Neurological Conditions. Min. Rev. Med. Chem 2003, 3, 785–787. [Google Scholar]
- Craigg, G.M.; Newman, D.J.; Weiss, R.B. Coral reefs, forests and thermal vents: The worldwide exploration of nature for novel antitumor agents. Semin. Oncol 1997, 24, 156–163. [Google Scholar]
- Bergmann, W.; Feeney, R.J. Contributions to the study of marine products. The nucleosides of sponges III. Spongothymidine and Spongouridine. J. Org. Chem 1985, 20, 1501–1507. [Google Scholar]
- Wolf, S.N.; Marion, J.; Stein, R.S. High dose cytosine arabinoside and daunorubicin as consolidation therapy for acute non lymphoblastic leukemia. Blood 1985, 65, 1407–1411. [Google Scholar]
- Moore, M. Activity of gemcitabine in patients with advanced pancreatic carcinoma: a review. Cancer 1996, 78(3), 633–637. [Google Scholar]
- Guchelaar, H.J.; Richel, Dj.; Van Knapen, A. Clinical, toxicological and pharmacological aspects of gemcitabine. Cancer. Treat. Rep 1996, 22, 15–31. [Google Scholar]
- Mayer, AMS; Gustaveson, KR. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int J Cancer 2003, 105, 291–299. [Google Scholar]
- Amador, M; Jimeno, J.; Paz Ares, L.; Cortés Funes, H.; Hidalgo, M. Progress in the development and acquisition of anticancer agents from marine sources. Ann Oncol 2003, 14(11), 1607–1615. [Google Scholar]
- Rinehart, K.L.; Holt, T.G.; Fregau, N.L. Ecteinascidins 729, 743, 745, 759ª, 759b and 770. Potent antitumor compounds from the Caribbean tunicate Ecteinascidia Turbinata. J Org Chem 1990, 55(15), 4515–4516. [Google Scholar]
- Jimeno, J.M.; Fairlocth, G.; Cameron, L.; Cameron, L.; Vega, E.; Meely, K.; Rinehart, K.; Fernandez-Sousa, JM. Progress in the acquisition of new marine derived anticancer compounds: development of ecteinascidin-743 (ET-743). Drugs of the Future 1996, 21(11), 1155–1165. [Google Scholar]
- Minuzzo, M; Marchini, S; Broggini, M; Faircloth, G; D’incalci, M; Mantovani, R. Interference of transcriptional activation by the anti-neoplastic drug ET-743. Proc.Natl.Acad.Sci.USA 2000, 97, 6780–6784. [Google Scholar]
- Jin, S; Gorfajn, B; Faircloth, G; Scotto, K. Ecteinascidin 743, a transcription-targeted chemotherapeutic that inhibits MDR1 activation. Proc.Natl.Acad.Sci.USA 2000, 97, 6775–6779. [Google Scholar]
- Friedman, D; Hu, Z; Kolb, EA; Gorfajn, B; Scotto, KW. Ecteinascidin-743 inhibits activated but not constitutive transcription. Cancer Res 2002, 62, 3377–3381. [Google Scholar]
- Kanzaki, A; Takebayashi, Y; Ren, XQ; Akiyama, S.; Bates, S.; Pommier, Y. Overcoming multidrug drug resistance in P-glycoprotein/MDR1-overexpressing cell lines by ecteinascidin 743. Mol Cancer Ther 2002, 1, 1327–1334. [Google Scholar]
- Damia, G; Silvestri, S; Carrassa, L; Filiberti, L; Faircloth, G T; Liberi, G; Foiani, M; D’Incalci, M. Unique pattern of ET-743 activity in different cellular systems with defined deficiencies in DNA-repair pathways. Int J Cancer 2001, 92, 583–588. [Google Scholar]
- Takebayashi, Y; Pourquier, P; Zimonjic, D B; Nakayama, K; Emmert, S; Ueda, T; Urasaki, Y; Kanzaki, A; Akiyama, S I; Popescu, N; Kraemer, K H; Pommier, Y. Antiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repair. Nat Med 2001, 7, 961–966. [Google Scholar]
- D’incalci, M; Colombo, T; Ubezio, P.; Nicoletti, I.; Giavazzi, R.; Erba, E.; Ferrarese, L.; Meco, D.; Riccardi, R.; Sessa, C.; Cavallini, E.; Jimeno, J.; Faircloth, G. The combination of yondelis and cisplatin is synergistic against human tumor xenografts. Eur J Cancer 2003, 39, 1920–1926. [Google Scholar]
- Sarries, C.; Haura, E.; Roig, B. Pharmacogenomic strategies for developing customized chemotherapy in non-small cell lung cancer. Pharmacogenomics 2002, 3(6), 763–780. [Google Scholar]
- Taamma, A; Misset, JL; Riofrio, M; Guzman, C; Brain, E; Lopez Lazaro, L; Rosing, H; Jimeno, J; Cvitkovic, E. Phase I and pharmacokinetic study of ecteinascidin-743, a new marine compound, administered as a 24-hour continuous infusion in patients with solid tumors. J Clin Oncol 2001, 19, 1256–1265. [Google Scholar]
- Ryan, DP; Supko, JG; Eder, JP; Seiden, M; Demetri, G; Lynch, TJ; Fischman, AJ; Davis, J; Jimeno, J; Clark, JW. Phase I and pharmacokinetic study of ecteinascidin 743 administered as a 72-hour continuous intravenous infusion in patients with solid malignancies. Clin Cancer Res 2001, 7, 231–242. [Google Scholar]
- Villalona-Calero, MA; Eckhardt, SG; Weiss, G; Hidalgo, M; Beijnen, JH; van Kesteren, C; Rosing, H; Campbell, E; Kraynak, M; Lopez-Lazaro, L; Guzman, C; Von Hoff, DD; Jimeno, J; Rowinsky, EK. A phase I and pharmacokinetic study of ecteinascidin-743 on a daily x 5 schedule in patients with solid malignancies. Clin Cancer Res 2002, 8, 75–85. [Google Scholar]
- Twelves, C.; Hoekman, K.; Bowman, A.; Vermorken, J.B.; Anthoney, A.; Smyth, J.; Van Kesteren, C.; Beijnen, J.H.; Uiters, J.; Wanders, J.; Gomez, J.; Guzmán, C.; Jimeno, J.; Hanauske, A. Phase I and pharmacokinetic study of Yondelis (Ecteinascidin 743; ET-743) administered as an infusion over 1h or 3h every 21 days in patients with solid tumors. Eur J Cancer 2003, 39, 1842–1851. [Google Scholar]
- Delaloge, S; Yovine, A; Taamma, A; Riofrio, M; Brain, E; Raymond, E; Cottu, P; Goldwasser, F; Jimeno, J; Misset, JL; Marty, M; Cvitkovic, E. Ecteinascidin-743: a marine-derived compound in advanced, pretreated sarcoma patients-preliminary evidence of activity. J Clin Oncol 2001, 19(5), 1248–1255. [Google Scholar]
- Demetri, G.; Manola, J.; Harmon, D.; Maki, R.; Seiden, R.; Supko, J.; Ryan, D.; Puchlaski, T.; Goss, G.; Merriam, P.; Waxman, A.; Slater, S.; Potter, A.; Quigley, T.; Lopez, T.; Sancho, MA.; Guzman, C.; Jimeno, J.; Garcia Carbonero, R. Ecteinascidin-743 (ET-743) induces durable responses and promising 1-year survival rates in soft tissue sarcomas (STS): Final results of Phase II and Pharmacokinetic studies in the U.S.A American Society of Clinical Oncology, San Francisco, CA, May 12 – 15; 2001. Procc. 352a. Abstract 1406.
- Le Cesne, A.; Blay, J.; Judson, I; Van Oosterom, A; Verweij, J; Radford, J; Lorigan, P; Rodenhuis, S; Donato, De; Paola, E; Van Glabbeke, M.; Jimeno, J.; Nielsen, O. ET-743 is an active drug in adult soft-tissue sarcoma: a STBSG-EORTC Phase II trial. American Society of Clinical Oncology, 37th Annual Meeting, San Francisco, CA, May 12 – 15; 2001. Procc. 353a. Abstract 1407. [Google Scholar]
- Yovine, A.; Riofrio, M.; Brain, E.; Blay, JY.; Kahatt, C.; Delaloge, S.; Beautier, L.; Cottu, P.; Jimeno, J.; Cvitkovic, E.; Misset, JL. Ecteinascidin-743 given as a 24 hour (H) intravenous continuos infusion (IVCI) every 3 weeks: results of a Phase II trial in patients (PTS) with pretreated soft tissue sarcomas (PSTS). American Society of Clinical Oncology. 37th Annual Meeting, San Francisco, CA, May 12 – 15; 2001. Proc. 363a. Abstract 1449. [Google Scholar]
- Jimeno, J.; Maki, R.; Casali, P.; Faircloth, G.; Martinez, N.; Nieto, A.; Cañigueral, S. Therapeutic impact of ET-743 (Yondelis®, Trabectidin) a new marine derived compound in Sarcoma. Current Opinion in Orthopedics. Submitted September 2003.
- Zelek, L.; Yovine, A.; Brain, E.; Jimeno, J.; Taamma, A.; Martin, C.; Spielmann, M.; Civtkovic, E.; Misset, JL. Ecteinascidin 743 in pretreated/advanced metastatic breast cancer patients: Preliminary results with the 24 hours infusion schedule. Procc. ASCO New Orleans 2000, 149, abst 592. [Google Scholar]
- Gómez, J.; López-Lázaro, L.; Guzmán, C.; González, A.; Misset, J.L.; Twelves, C.; Bowman, A.; Hoekman, K.; Villalona, M.; Ryan, D.; Pazares, L.; Jimeno, J. Identification of biochemical parameters that predict the onset of severe toxicities in patients treated with ET-743. ASCO New Orleans 2000. Procc. Abst 727:187ª. [Google Scholar]
- Lopez Martin, J.A.; Nieto, A.; Demetri, G.; Misset, JL.; Ray-Coquard, I.; Guzman, C.; Sancho, MA.; Parra, MC.; Ibarra, I.; Millan, S.; Martín, C.; Ruiz, A.; De Alvaro, J.; Lopez-Lazaro, L.; Gomez, J.; Jimeno, J. Safety profile of ecteinascidin-743 (ET-743) in phase II clinical trials (CT)in adult patients with solid tumors. Proc Am Soc Clin Oncol 2002, 21, 96a. [Google Scholar]
- Colombo, N.; Capri, G.; Bauer, J.; Noberasco, C.; De Braud, F.; Grasselli, G.; Jimeno, J.; Ruiz, A.; Corradino, I.; Marsoni, S.; Sessa, C. Phase II and Pharmacokinetics study of 3 hour infusion of ET743 in ovarian cancer patients failing platinum-taxanes. ASCO 38th Annual Meeting, Orlando, Florida, May 18–21; 2002. Proc.. 21, Abstract 880. p. 221a. [Google Scholar]
- Casali, P.; Casanova, N.; Dileo, P.; Ferrari, S.; Bacci, G.; Picci, P.; Marsoni, S.; Jimeno, J.; Riccardi, R.; Gianni, L. Activity of Ecteinascidin 743 (ET-trabectedin 743) 3 h infusion in adult and childhood small round cell sarcomas. ASCO 39th Annual Meeting, Chicago IL, May 31-June 3; 2003. Proc Vol. 22 Abs. 962. p. 240. [Google Scholar]
- Forouzesh, B.; Hidalgo, M.; Denis, L.; Schwart, G.; Hammond, L.; Monroe, P.; Guzman, C.; Lopez-Lazaro, L.; Supko, J.; Jimeno, J.; Rowinsky, E. phase I and pharmacokinetic study of the marinederived DNA minor groove binder ET-743 on a weekly x3 every-4-week schedule in patients with advanced solid malignancies. Proc of AACR-NCI-EORTC International Conference on Molecul targets and cancer therapeutics: Discovery, Biology and Clinical applications, Miami Beach, October 29-November 2; 2001; p. 42. [Google Scholar]
- Grasselli, G.; Malossi, A.; Marsoni, S.; Colombo, N.; Perez, C.; Zucchetti, M.; D’incalci, M.; Lopez Martin, JA.; Jimeno, J; Capri, G.; Raspagliesi, F.; Curigliano, G.; Gianni, L.; Sessa, C. Phase I and pharmacokinetic (PK) study of ecteinascidin-743 (ET, Trabectedin) and cisplatin (P) combination in pre-treated patients (pts) with selected advanced solid tumors. Proc Am Soc Clin Oncol 2003, 22, N°. 542. [Google Scholar]
- Rinehart, K.L.; Gloer, J.B.; Cook, J.C.; Mizsak, S.A.; Scohill, T.A. Structures of the Didemnins, Antiviral and Cytotoxic Depsipeptides from a Caribbean Tunicate. J Am Chem Soc 1981, 103, 1857–1859. [Google Scholar]
- Erba, E.; Bassano, L.; Di Liberti, G.; Muradore, I.; Chiorino, G.; Ubezio, P.; Vignati, S.; Codegoni, A.; Desiderio, A.; Faircloth, G.; Jimeno, J.; D’Incalci, M. Cell Cycle phase perturbations and apoptosis in tumour cells induced by aplidine. Br J Cancer 2002, 86, 1510–17. [Google Scholar]
- Broggini, M; Marchini, S.; Borsott, P.; Galliera, E.; Erba, E.; Taraboletti, G.; Giavazzi, R.; Jimeno, J.; Faircloth, G.; D’incalci, M. Aplidine, a new anticancer agent of marine origin, inhibits vascular endothelial growth factor (VEGF) secretion and blocks VEGF-VEGFR-1 (flt-1) autocrine loop in human leukemia cells MOLT-4. Leukemia 2003, 17, 52–9. [Google Scholar]
- Anthoney, A.; Paz-Ares, L.; Twelves, C.; Cortes-Funes, H.; Kaye, S.; Pronk, L.; Celli, N.; Lopez-Lazaro, L.; Guzman, C.; Jimeno, J. Phase I and Pharmacokinetic (PK) Study of APL (APL) Using a 24-Hour, Weekly Schedule. Proc Am Soc Clin Oncol 2000. abstract #734. [Google Scholar]
- Ciruelos, E.M.; Twelves, C.; Dominguez, M.J.; Mckay, H.; Anthony, A; Castellanos, D.; Bezares, S.; Ruiz, A.; Lopez-Lazaro, L.; Jimeno, J.; Celli, C.; Cortes-Funes, H.; Paz-Ares, L. Phase I clinical and pharmacokinetic study of the marine compound APL (APL) administered as a 3 hour infusion every 2 weeks. Proc Am Soc Clin Oncol 2002. abstract # 422. [Google Scholar]
- Bowman, A.; Izquierdo, M.A.; Jodrell, D.; Martinez, M.; Cicchella, B.; Jimeno, J.; Guzman, C.; Germa-Lluch, J.; Celli, N.; Smyth, J. Phase I Clinical and Pharmacokinetic (PK) Study of the Marine Compound APL (APL), administered as a 1 Hour Weekly Infusion. Proc Am Soc Clin Oncol 2001. abstract #476. [Google Scholar]
- Armand, J.P.; Ady-Vago, N.; Faivre, S.; Chieze, S.; Baudin, E.; Ribrag, V.; Lecot, F.; Iglesias, L.; López-Lázaro, L.; Guzmán, C.; Jimeno, J.; Ducreux, M.; Le Chevalier, T.; Raymond, E. Phase I and Pharmacokinetic Study of APL Given as a 24-Hour Continuous Infusion Every Other Week (q2w) in Patients (pts) with Solid Tumor (ST) and Lymphoma (NHL). Proc Am Soc Clin Oncol 2001. abstract #477. [Google Scholar]
- Maroun, J.A.; Goel, R.; Stewart, D.J.; Tomiak, E.; Belanger, K.; Soulieres, D.; Charpentier, D.; Seymour, L.; Matthews, S.; Jimeno, J.; Guzman, C. Phase I Study of APL in a 5 Day Bolus Q 3 Weeks in Patients with Solid Tumors and Lymphomas. Proc Am Soc Clin Oncol 2001. abstract #2082. [Google Scholar]
- Bresters, D.; Broekhuizen, A.; Faircloth, G.; Jimeno, J.; Kaspers, G. In vitro cytotoxicity of Aplidine and cross-resistance with other cytotoxic drugs in childhood leukemic and normal bone marrow and blood samples; a rational basis for clinical development. Leukemia 2003, 1–6. [Google Scholar]
- Erba, E.; Serafini, M.; Gaipa, G.; Tognon, G.; Marchini, S.; Muradore, I.; Celli, N.; Rotilio, D.; Broggini, M.; Jimeno, J.; Faircloth, G.; Biondi, A.; D’incalci, M. The effect of Aplidine in acute lymphoblastic leukaemia cells. Br J Cancer Suppl 2003, 89, 763–773. [Google Scholar]
- Raymond, E; Paz-Ares, L; Izquierdo, MA; Pardo, B; Ciruelos, E; Baudin, E; Domínguez, MJ; Martinez, M; Jimeno, J; Lopez-Martin, JA. Activity of APL, a new marine compound, against medullary thyroid carcinoma (MTC): Phase I trials as screening tools for rare tumors. Ann Oncol. 2002, 13 Suppl 5, 22. [Google Scholar]
- Hamann, MT; Otto, CS; Scheuer, PJ. Kahalalides: Bioactive peptides from a marine mollusk Elysia rufescens and its algal diet Bryopsis sp. J. Org. Chem 1996, 61, 6594–660. [Google Scholar]
- Hamann, MT; Scheuer, PJ. Kahalalide F: A bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J Am Chem Soc 1993, 115, 5825–5826. [Google Scholar]
- García-Rocha, M; Bonay, P; Avila, J. The antitumor compound Kahalalide F acts on cell lysosomes. Cancer Lett 1996, 99, 43–50. [Google Scholar]
- Suarez, Y.; Gonzalez, L.; Cuadrado, A.; Berciano, M.; Lafarga, M.; Muñoz, A. Kahalalide F, a new marine derived compound induces oncosis in human prostate and breast cancer cells. Mol. Cancer Therapeutics 2003, 863–872. [Google Scholar]
- Supko, J.G; Lu, H.; Jimeno, J.M.; Grant, W.; Faircloth, G.T. Preclinical pharmacology studies with the marine natural product Kahalalide F. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, Washington D.C., Abstract no. 315. November 16–19; 1999. [Google Scholar]
- Ciruelos, E.; Trigo, JM.; Pardo, B.; Paz-Ares, L.; Estaun, N.; Cuadra, C.; Domínguez, MJ.; Marin, A.; Ruiz, A.; Jimeno, J.; Izquierdo, MA. A phase I clinical and pharmacokinetic (PK) study with KahalalideF (KF) in patients (pts) with advanced solid tumours (AST) with a continuous weekly (W) 1-hour iv infusion schedule. In European Journal of Cancer, 14th NCI-EORTC-AACR Congress, Frankfurt, Germany, 19–22 November; 2002; 38 Supplement 7, p. S33, Abstract 95. [Google Scholar]
- Schellens, J.H.M.; Rademakerlakhai, J.M.; Horenblas, S.; Meinhardt, W.; Stokvis, E.; De Reijke, T.M.; Jimeno, J.; Lopez-Lazaro, L.; Lopez-Martin, J.A.; Beijnen, J.H. Phase I and pharmacokinetic study of kahalalide F in patients with advanced androgen resistant prostate cancer. ASCO 38th Annual Meeting, Orlando, Florida, May 18–21 2002. Proc.113a, Vol., 21, Abstract 451.
- Cuevas, C; Perez, M; Martin, M J; Chicharro, J L; Fernandez-Rivas, C; Flores, M; Francesch, A; Gallego, P; Zarzuelo, M; de La Calle, F; Garcia, J; Polanco, C; Rodriguez, I; Manzanares, I. Síntesis of Ecteinascidin ET-743 and phthalascidin PT-650 from syano safracin B. Org. Lett 2000, 2, 2245–2548. [Google Scholar]
- Joug, G.; Gonzalez, L.; Albericio, F; Lloyd-Williams, P; Giralt, E. Total síntesis of dehydro didemnin B. Use of uranium and phosphonium salt coupling reagents in peptide synthesis in solution. J. Org. Chem 1997, 62, 354–366. [Google Scholar]
- Lopez Macia, A.; Jiménez, JC; Royo, M.; Giralt, E.; Albericio, F. Synthesis and structure determination of Kahalalide F. Am. Chem Society 2001, 123, 11398–11401. [Google Scholar]
- Cuadros, R.; Montejo, De; Garcini, E.; Wandosell, F.; Faircloth, G.; Fernández Sousa, JM; Avila, J. The marine compound Spisulosine, and inhibitor of cell proliferation promotes the disassembly of actin stress fibers. Cancer Lett 2000, 152, 23–29. [Google Scholar]
- Jimeno, J.; García-Grávalos, D.; Ávila, J.; Smith, B.; Grant, W.; Faircloth, G.T. ES-285, a marine natural product with activity against solid tumors. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, Washington, D.C., November 16–19, 1999. Abstract no. 314.
- Sanchez Beato, M.; Moneo, V; Martínez, N; Fernández, I; Carnero, A; Piris, MA; Jimeno, J. Molecular pharmacodynamic (MPD) effects induced by ET-743 (Yondelis) in human sarcoma cells (HSC). Procc International Conference on Applied Genomics – 9th ESCAP/16th ISDQP Meeting, Amsterdam, The Netherlands, October 1–4, 2003. Abstract C-1465.
© 2004 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Jimeno, J.; Faircloth, G.; Sousa-Faro, J.F.; Scheuer, P.; Rinehart, K. New Marine Derived Anticancer Therapeutics ─ A Journey from the Sea to Clinical Trials. Mar. Drugs 2004, 2, 14-29. https://doi.org/10.3390/md201014
Jimeno J, Faircloth G, Sousa-Faro JF, Scheuer P, Rinehart K. New Marine Derived Anticancer Therapeutics ─ A Journey from the Sea to Clinical Trials. Marine Drugs. 2004; 2(1):14-29. https://doi.org/10.3390/md201014
Chicago/Turabian StyleJimeno, J., G. Faircloth, JM Fernández Sousa-Faro, P. Scheuer, and K. Rinehart. 2004. "New Marine Derived Anticancer Therapeutics ─ A Journey from the Sea to Clinical Trials" Marine Drugs 2, no. 1: 14-29. https://doi.org/10.3390/md201014




