Total Synthesis of Pulmonarin B and Design of Brominated Phenylacetic Acid/Tacrine Hybrids: Marine Pharmacophore Inspired Discovery of New ChE and Aβ Aggregation Inhibitors
Abstract
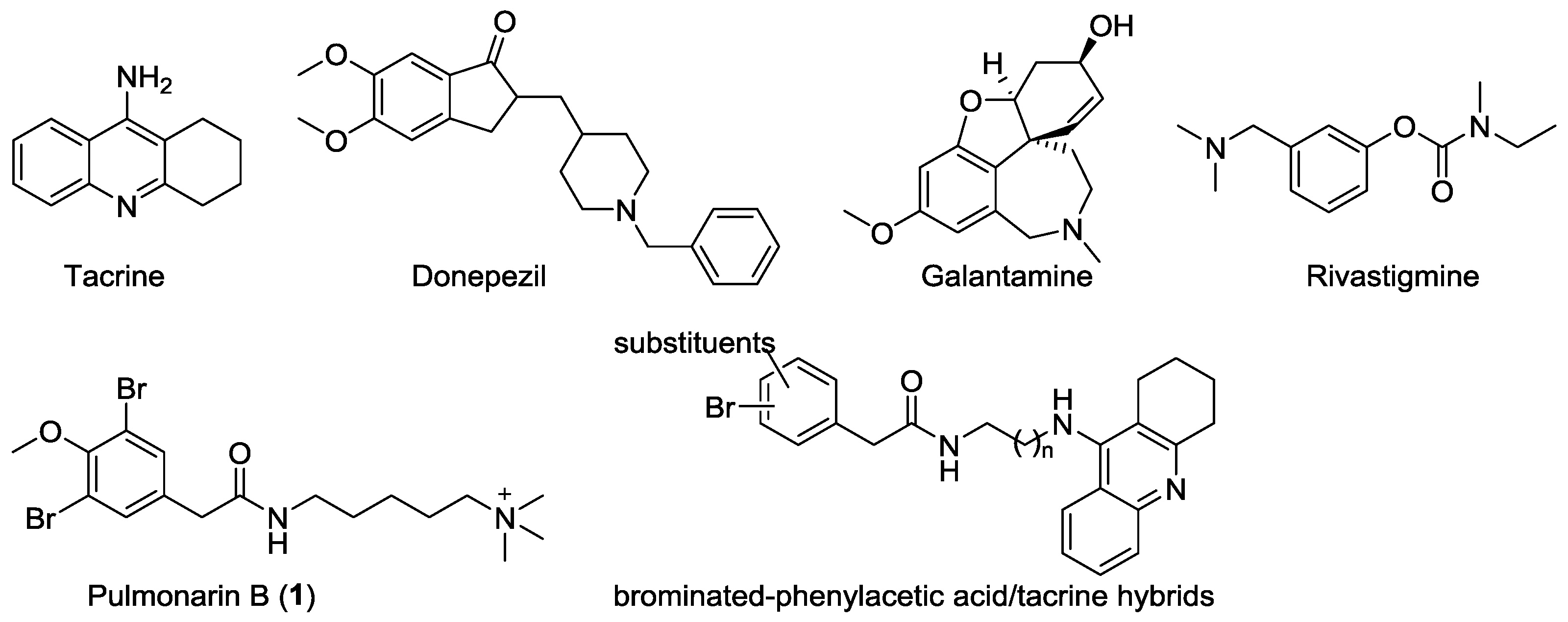
:1. Introduction
2. Results and Discussion
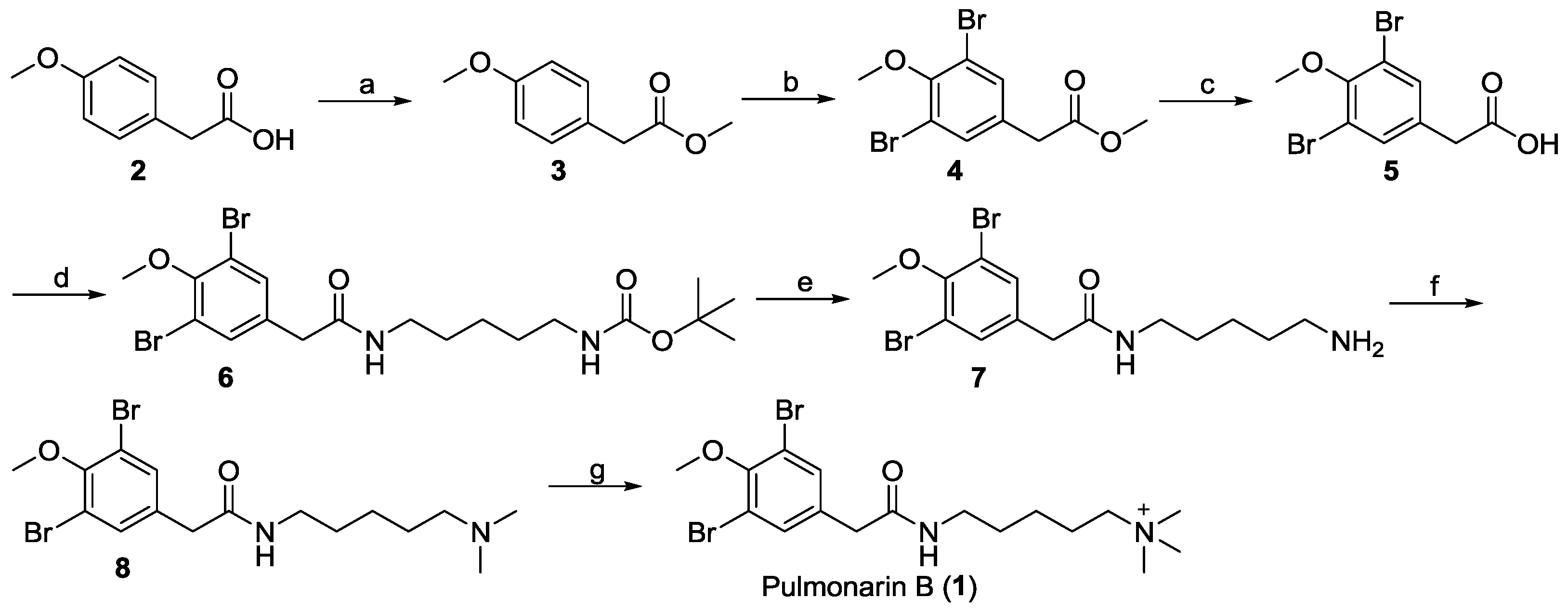
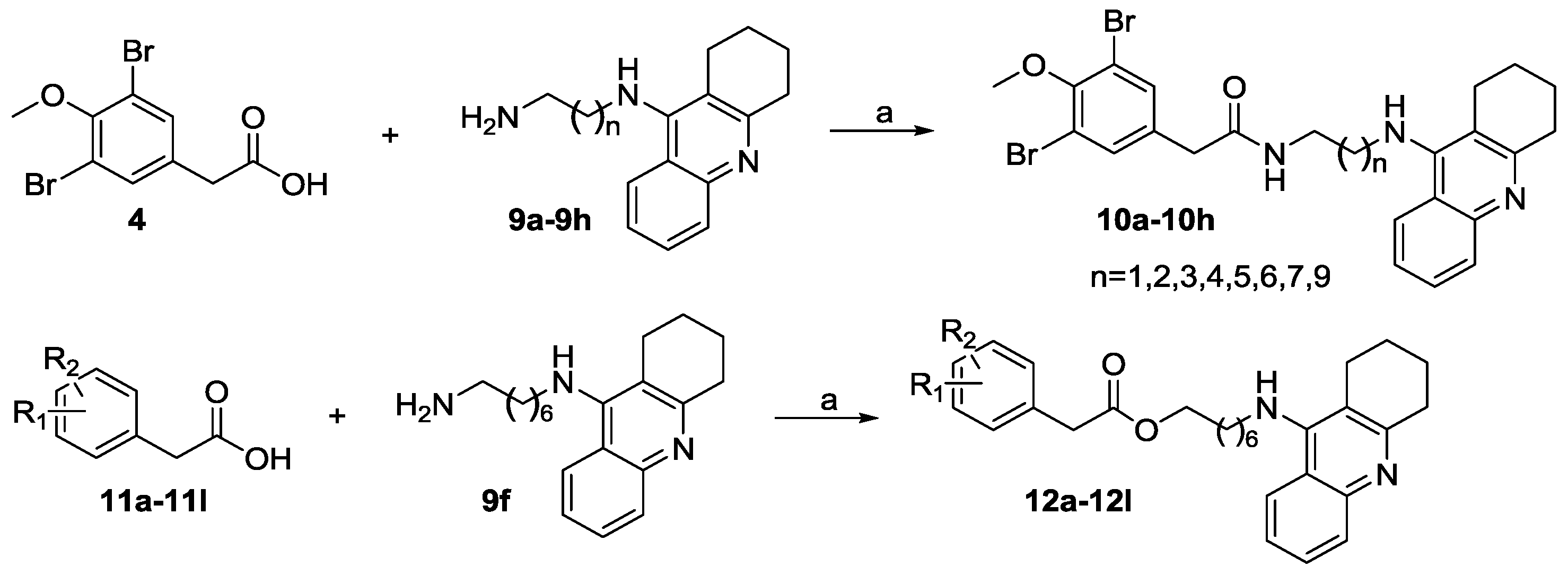
2.1. Chemistry
2.2. In Vitro Inhibition of AChE and BChE, and Structure–Activity Relationship (SAR) Analysis
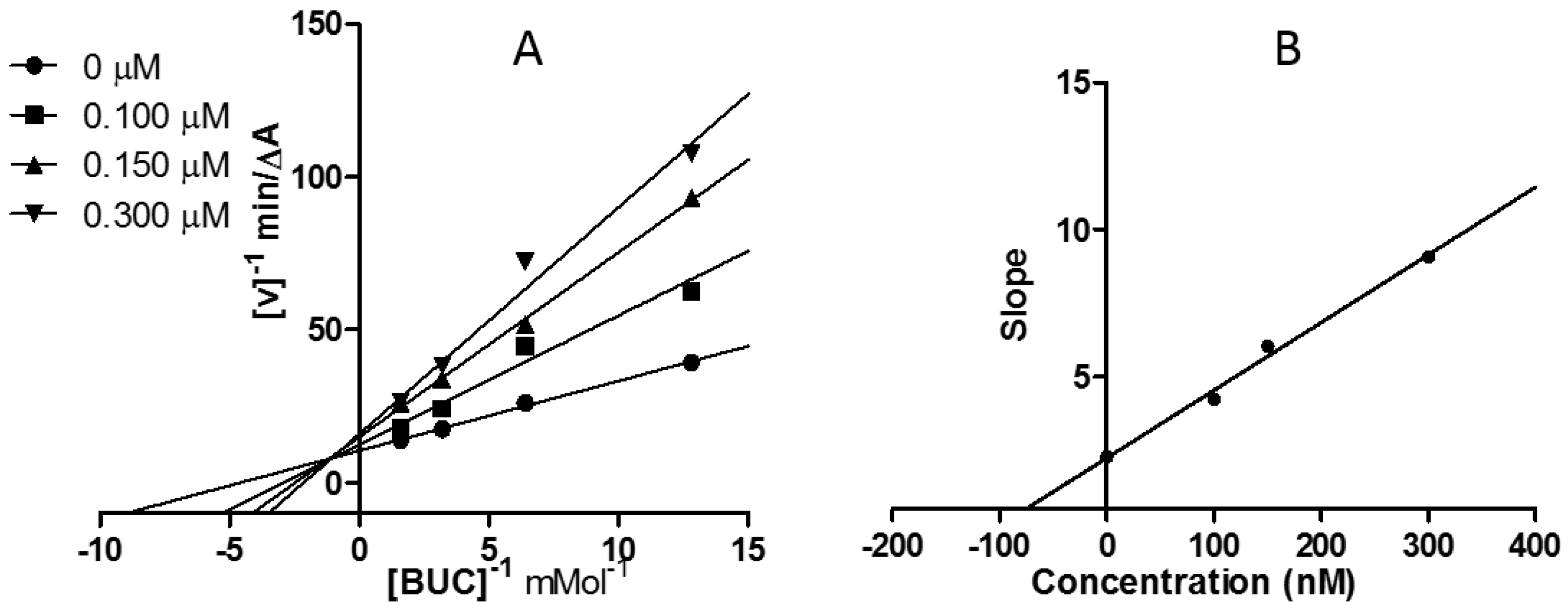
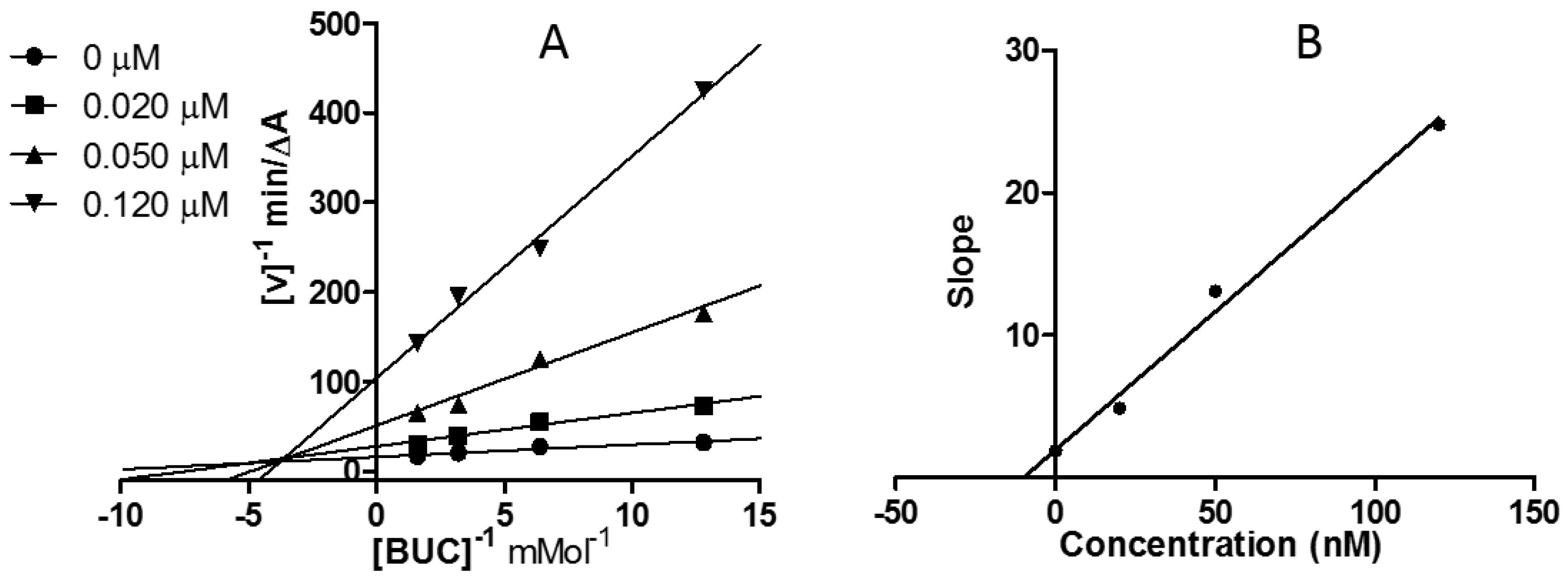
2.3. Kinetic Study of AChE and BChE Inhibition
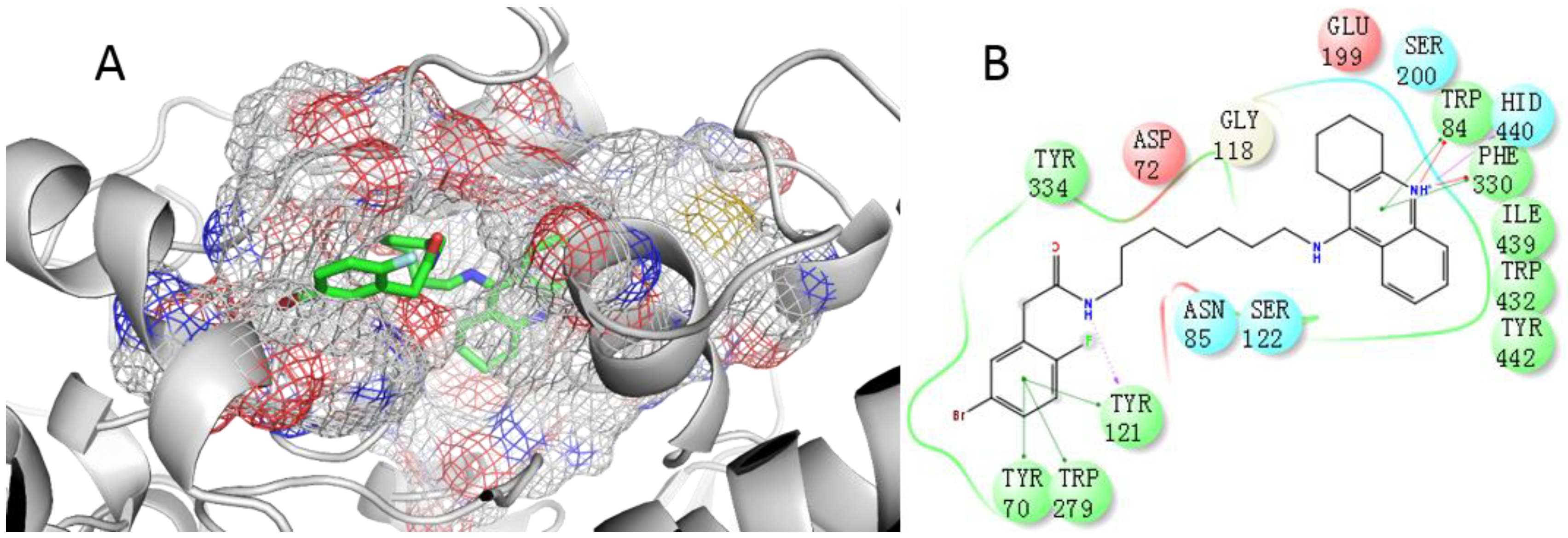
2.4. Docking Study
2.5. Inhibition of Self-Induced and AChE-Induced Aβ Aggregation
2.6. In Vitro Cytotoxicity toward HepG2 Cells
3. Materials and Methods
3.1. General Information
3.2. Chemistry
General Procedures for the Synthesis of 10a–10h and 12a–12l
3.3. AChE/BChE Inhibitory Assay
3.4. Kinetic Assay
3.5. Molecular Docking
3.6. Determination of the Inhibitory Potency on Self-Aβ1–42 Aggregation
3.7. Determination of the Inhibitory Potency on Aβ1–42 Aggregation Induced by AChE
3.8. Cytotoxicity Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thiratmatrakul, S.; Yenjai, C.; Waiwut, P.; Vajragupta, O.; Reubroycharoen, P.; Tohda, M.; Boonyarat, C. Synthesis, biological evaluation and molecular modeling study of novel tacrine-carbazole hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 75, 21–30. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Jakk, S.L.; Senthil, V.; Yasam, V.R.; Chandrasekar, M.J.N.; Vijayaraghavan, C. The Blood Brain Barrier and its Role in Alzheimer’s Therapy: An Overview. Curr. Drug Targets 2018, 19, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.F.; Huang, Y.D.; Zhang, C.; Wu, X.N.; Zhou, Q.; Wu, D.; Wu, Y.; Luo, H.B. Discovery of Novel Pyrazolopyrimidinone Derivatives as Phosphodiesterase 9A Inhibitors Capable of Inhibiting Butyrylcholinesterase for Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2017, 8, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76 Pt A, 27–50. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Yu, Q.; Zhu, X.; Holloway, H.W.; Perry, T.; Lee, B.; Ingram, D.K.; Lahiri, D.K. A new therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- AnandSingh, P.B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar]
- Feng, B.; Li, X.P.; Xia, J.; Wu, S. Discovery of novel isoflavone derivatives as AChE/BuChE dual-targeted inhibitors: Synthesis, biological evaluation and molecular modeling. J. Enzyme Inhib. Med. Chem. 2017, 32, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.S.; Fu, Y.; Zhang, L.; Gong, J.X.; Wang, Z.Z.; Xiao, W.; Zhang, H.Y.; Guo, Y.W. Synthesis and biological evaluation of novel marine-derived indole-based 1,2,4-oxadiazoles derivatives as multifunctional neuroprotective agents. Bioorg. Med. Chem. Lett. 2015, 25, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, H.; Chen, Y.; Lin, H.; Li, Q.; Mo, J.; Bian, Y.; Pei, Y.; Sun, H. Synthesis, pharmacology and molecular docking on multifunctional tacrine-ferulic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2018, 33, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Dai, Y.C.; Li, N.G.; Dong, Z.X.; Gu, T.; Shi, Z.H.; Xue, X.; Tang, Y.P.; Duan, J.A. Novel multitarget-directed tacrine derivatives as potential candidates for the treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2017, 32, 572–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Alexandre Muehlmann, L.; Jiang, C.S.; Guo, Y.W. Synthesis and Structural Modification of Marine Natural Products. Molecules 2017, 22, 882. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Biological Activity of Recently Discovered Halogenated Marine Natural Products. Mar. Drugs 2015, 13, 4044–4136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, R.; Swanson, G.T. Recent progress in neuroactive marine natural products. Nat. Prod. Rep. 2014, 31, 273–309. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Zhang, J.; Rodrigues, M.C.; Ding, D.J.; Longo, J.P.; Azevedo, R.B.; Muehlmann, L.A.; Jiang, C.S. Synthesis and evaluation of novel 1,2,3-triazole-based acetylcholinesterase inhibitors with neuroprotective activity. Bioorg. Med. Chem. Lett. 2016, 26, 3881–3885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.S. Synthesis and evaluation of coumarin/piperazine hybrids as acetylcholinesterase inhibitors. Med. Chem. Res. 2018, 27, 1717–1727. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.C.; Song, J.L.; Cheng, Z.Q.; Sun, J.Z.; Jiang, C.S. Synthesis and evaluation of coumarin/1,2,4-oxadiazoles hybrids as selective BChE inhibitors with neuroprotective activity. J. Asian Nat. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.; Svenson, J.; Sepčić, K.; Trembleau, L.; Engqvist, M.; Andersen, J.H.; Jaspars, M.; Stensvåg, K.; Haug, T. Isolation and Synthesis of Pulmonarins A and B, Acetylcholinesterase Inhibitors from the Colonial Ascidian Synoicum pulmonaria. J. Nat. Prod. 2014, 77, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeřábek, J.; Uliassi, E.; Guidotti, L.; Korábečný, J.; Soukup, O.; Sepsova, V.; Hrabinova, M.; Kuča, K.; Bartolini, M.; Peña-Altamira, L.E.; et al. Tacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Nepovimova, E.; Korabecny, J.; Dolezal, R.; Babkova, K.; Ondrejicek, A.; Jun, D.; Sepsova, V.; Horova, A.; Hrabinova, M.; Soukup, O.; et al. Tacrine-trolox hybrids: A novel class of centrally active, nonhepatotoxic multi-target-directed ligands exerting anticholinesterase and antioxidant Activities with low in vivo toxicity. J. Med. Chem. 2015, 58, 8985–9003. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.; Mo, J.; Yang, H.; Jiang, X.; Lin, H.; Gu, K.; Pei, Y.; Wu, L.; Tan, R.; et al. Synthesis and bioevaluation of new tacrine-cinnamic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2018, 33, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Delogu, G.L.; Matos, M.J.; Fanti, M.; Era, B.; Medda, R.; Pieroni, E.; Fais, A.; Kumar, A.; Pintus, F. 2-Phenylbenzofuran derivatives as butyrylcholinesterase inhibitors: Synthesis, biological activity and molecular modeling. Bioorg. Med. Chem. Lett. 2016, 26, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, E.H.; Brumshtein, B.; Greenblatt, H.M.; Wong, D.M.; Shaya, D.; Williams, L.D.; Carlier, P.R.; Pang, Y.P.; Silman, I.; Sussman, J.L. Complexes of alkylene-linked tacrine dimers with Torpedo californica acetylcholinesterase: Binding of bis5-tacrine produces a dramatic rearrangement in the active-site gorge. J. Med. Chem. 2006, 49, 5491–5500. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Talesa, T.N. Acetylcholinesterase in Alzheimer’s Disease. Mech. Ageing Dev. 2001, 122, 1961–1969. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and and Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Fang, X.; Gou, S.; Lupp, A.; Lenhardt, I.; Sun, Y.; Huang, Z.; Chen, Y.; Zhang, Y.; Fleck, C. Design, synthesis and biological evaluation of D-ring opened galantamine analogs as multifunctional anti-Alzheimer agents. Eur. J. Med. Chem. 2014, 76, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Baleh, L.; Nadri, H.; Moradi, A.; Bukhari, S.N.A.; Shakibaie, M.; Jafari, M.; Golshani, M.; Homayouni Moghadam, F.; Firoozpour, L.; Asadipour, A.; et al. New racemic annulated pyrazolo[1,2-b]phthalazines as tacrine-like AChE inhibitors with potential use in Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 280–289. [Google Scholar] [CrossRef] [PubMed]
| Compound |  |  | EeAChE 2 | eqBChE 3 |
|---|---|---|---|---|
| 1 | 37.02 ± 2.11 | 30.70 ± 1.44 | ||
| 10a | 1 | 0.880 ± 0.074 | 0.230 ± 0.010 | |
| 10b | 2 | 0.771 ± 0.027 | 0.233 ± 0.013 | |
| 10c | 3 | 1.045 ± 0.161 | 0.195 ± 0.011 | |
| 10d | 4 | 0.764 ± 0.016 | 0.100 ± 0.014 | |
| 10e | 5 | 0.686 ± 0.045 | 0.054 ± 0.006 | |
| 10f | 6 | 0.314 ± 0.010 | 0.053 ± 0.007 | |
| 10g | 7 | 0.427 ± 0.016 | 0.325 ± 0.004 | |
| 10h | 9 | 0.638 ± 0.026 | 0.516 ± 0.037 | |
| 12a | R1 = 2-Br, R2 = 4-OMe | 0.324 ± 0.033 | 0.140 ± 0.004 | |
| 12b | R1 = H, R2 = 4-OMe | 0.750 ± 0.054 | 0.694 ± 0.003 | |
| 12c | R1 = 3-Br, R2 = 4-OMe | 0.607 ± 0.045 | 0.152 ± 0.006 | |
| 12d | R1 = 2-Br, R2 = H | 0.304 ± 0.004 | 0.091 ± 0.017 | |
| 12e | R1 = 3-Br, R2 = H | 0.724 ± 0.003 | 0.149 ± 0.021 | |
| 12f | R1 = 4-Br, R2 = H | 0.597 ± 0.028 | 0.214 ± 0.004 | |
| 12g | R1 = 2-Br, R2 = 5-Br | 0.696 ± 0.052 | 0.062 ± 0.006 | |
| 12h | R1 = 3-Br, R2 = 5-Br | 0.729 ± 0.021 | 0.204 ± 0.011 | |
| 12i | R1 = 3-Br, R2 = 4-F | 0.748 ± 0.017 | 0.049 ± 0.004 | |
| 12j | R1 = 5-Br, R2 = 2-F | 0.182 ± 0.006 | 0.064 ± 0.006 | |
| 12k | R1 = 2-Br, R2 = 5-Cl | 0.383 ± 0.054 | 0.104 ± 0.001 | |
| 12l | R1 = R2 = H | 0.760 ± 0.092 | 0.680 ± 0.054 | |
| Tacrine | 0.159 ± 0.007 | 0.046 ± 0.002 |
| Compounds | Inhibition of Aβ Aggregation (%) | |
|---|---|---|
| Self-Induced 1 | AChE-Induced 2 | |
| 1 | 29.78 ± 1.45 | 27.60 ± 1.96 |
| 12j | 32.37 ± 0.62 | 47.73 ± 4.35 |
| Tacrine | NT 3 | 21.84 ± 1.60 |
| Donepezil | 17.95 ± 0.77 | 22.42 ± 2.56 |
| Galantamine | 1.25 ± 0.46 | 3.58 ± 1.42 |
| Compound | IC50 1 |
|---|---|
| 1 | >80 2 |
| 12j | >80 |
| Tacrine | 38.87 ± 0.53 |
| Donepezil | 37.92 ± 1.46 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.-Q.; Song, J.-L.; Zhu, K.; Zhang, J.; Jiang, C.-S.; Zhang, H. Total Synthesis of Pulmonarin B and Design of Brominated Phenylacetic Acid/Tacrine Hybrids: Marine Pharmacophore Inspired Discovery of New ChE and Aβ Aggregation Inhibitors. Mar. Drugs 2018, 16, 293. https://doi.org/10.3390/md16090293
Cheng Z-Q, Song J-L, Zhu K, Zhang J, Jiang C-S, Zhang H. Total Synthesis of Pulmonarin B and Design of Brominated Phenylacetic Acid/Tacrine Hybrids: Marine Pharmacophore Inspired Discovery of New ChE and Aβ Aggregation Inhibitors. Marine Drugs. 2018; 16(9):293. https://doi.org/10.3390/md16090293
Chicago/Turabian StyleCheng, Zhi-Qiang, Jia-Li Song, Kongkai Zhu, Juan Zhang, Cheng-Shi Jiang, and Hua Zhang. 2018. "Total Synthesis of Pulmonarin B and Design of Brominated Phenylacetic Acid/Tacrine Hybrids: Marine Pharmacophore Inspired Discovery of New ChE and Aβ Aggregation Inhibitors" Marine Drugs 16, no. 9: 293. https://doi.org/10.3390/md16090293
APA StyleCheng, Z.-Q., Song, J.-L., Zhu, K., Zhang, J., Jiang, C.-S., & Zhang, H. (2018). Total Synthesis of Pulmonarin B and Design of Brominated Phenylacetic Acid/Tacrine Hybrids: Marine Pharmacophore Inspired Discovery of New ChE and Aβ Aggregation Inhibitors. Marine Drugs, 16(9), 293. https://doi.org/10.3390/md16090293






