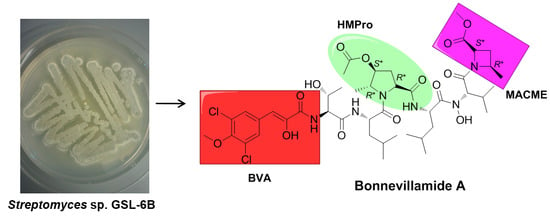
Bonnevillamides, Linear Heptapeptides Isolated from a Great Salt Lake-Derived Streptomyces sp.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Biosynthetic Proposal
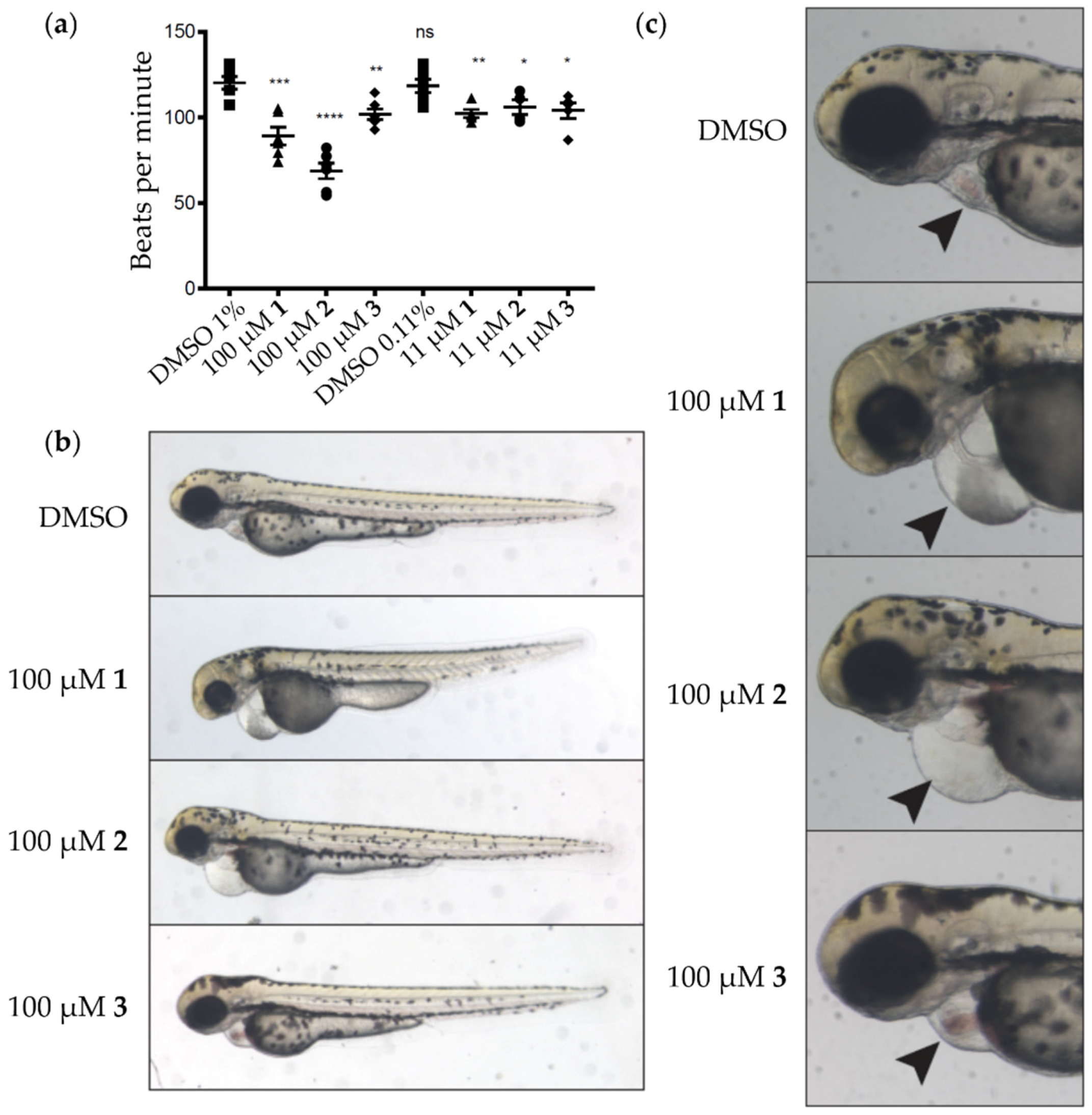
2.3. Bioactivity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Isolation and Fermentation of Streptomyces sp. GSL-6B
3.3. Extraction and Isolation
3.4. Advanced Marfey’s Anslysis
3.5. Zebrafish Assays
3.6. Heartrate Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Skropeta, D. Deep-sea natural products. Nat. Prod. Rep. 2008, 25, 1131–1166. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, Y.; Lee, S.H.; Oh, K.B.; Lee, S.K.; Shin, J.; Oh, D.C. Salternamides A–D from a Halophilic Streptomyces sp. Actinobacterium. J. Nat. Prod. 2015, 78, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ha, T.K.; Oh, W.K.; Shin, J.; Oh, D.C. Antiviral Indolosesquiterpenoid Xiamycins C–E from a Halophilic Actinomycete. J. Nat. Prod. 2016, 79, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.C.; Zabriskie, T.M.; McPhail, K.L. Deep-sea hydrothermal vents: Potential hot spots for natural products discovery? J. Nat. Prod. 2010, 73, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K. Culturability and secondary metabolite diversity of extreme microbes: Expanding contribution of deep sea and deep-sea vent microbes to natural product discovery. Mar. Biotechnol. 2011, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.E.; Brimble, M.A. Molecules derived from the extremes of life. Nat. Prod. Rep. 2009, 26, 44–71. [Google Scholar] [CrossRef] [PubMed]
- Tazi, L.; Breakwell, D.P.; Harker, A.R.; Crandall, K.A. Life in extreme environments: Microbial diversity in Great Salt Lake, Utah. Extremophiles 2014, 18, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.J.; Rompato, G.; Crowl, T.A.; Weimer, B.C.; Pfrender, M.E. Phylogenetic distance in Great Salt Lake microbial communities. Aquat. Microb. Ecol. 2011, 64, 267–273. [Google Scholar] [CrossRef]
- Almeida-Dalmet, S.; Sikaroodi, M.; Gillevet, P.M.; Litchfield, C.D.; Baxter, B.K. Temporal Study of the Microbial Diversity of the North Arm of Great Salt Lake, Utah, U.S. Microorganisms 2015, 3, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Meuser, J.E.; Baxter, B.K.; Spear, J.R.; Peters, J.W.; Posewitz, M.C.; Boyd, E.S. Contrasting patterns of community assembly in the stratified water column of Great Salt Lake, Utah. Microb. Ecol. 2013, 66, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Elkobi-Peer, S.; Faigenbaum, R.; Carmeli, S. Bromine- and chlorine-containing aeruginosins from Microcystis aeruginosa bloom material collected in Kibbutz Geva, Israel. J. Nat. Prod. 2012, 75, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Elkobi-Peer, S.; Singh, R.K.; Mohapatra, T.M.; Tiwari, S.P.; Carmeli, S. Aeruginosins from a Microcystis sp. bloom material collected in Varanasi, India. J. Nat. Prod. 2013, 76, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Ikai, Y.; Mayumi, T.; Oka, H.; Suzuki, M.; Harada, K. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: Elucidation of limitations of Marfey’s method and of its separation mechanism. Anal. Chem. 1997, 69, 3346–3352. [Google Scholar] [CrossRef]
- Fujii, K.; Ikai, Y.; Oka, H.; Suzuki, M.; Harada, K. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: Combination of Marfey's method with mass spectrometry and its practical application. Anal. Chem. 1997, 69, 5146–5151. [Google Scholar] [CrossRef]
- Lin, Z.; Flores, M.; Forteza, I.; Henriksen, N.M.; Concepcion, G.P.; Rosenberg, G.; Haygood, M.G.; Olivera, B.M.; Light, A.R.; Cheatham, T.E.; et al. Totopotensamides, polyketide-cyclic peptide hybrids from a mollusk-associated bacterium Streptomyces sp. J. Nat. Prod. 2012, 75, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: Logic, machinery, and mechanisms. Chem. Rev. 2006, 106, 3468–3496. [Google Scholar] [CrossRef] [PubMed]
- Kyndt, J.A.; Meyer, T.E.; Cusanovich, M.A.; Van Beeumen, J.J. Characterization of a bacterial tyrosine ammonia lyase, a biosynthetic enzyme for the photoactive yellow protein. FEBS Lett. 2002, 512, 240–244. [Google Scholar] [CrossRef]
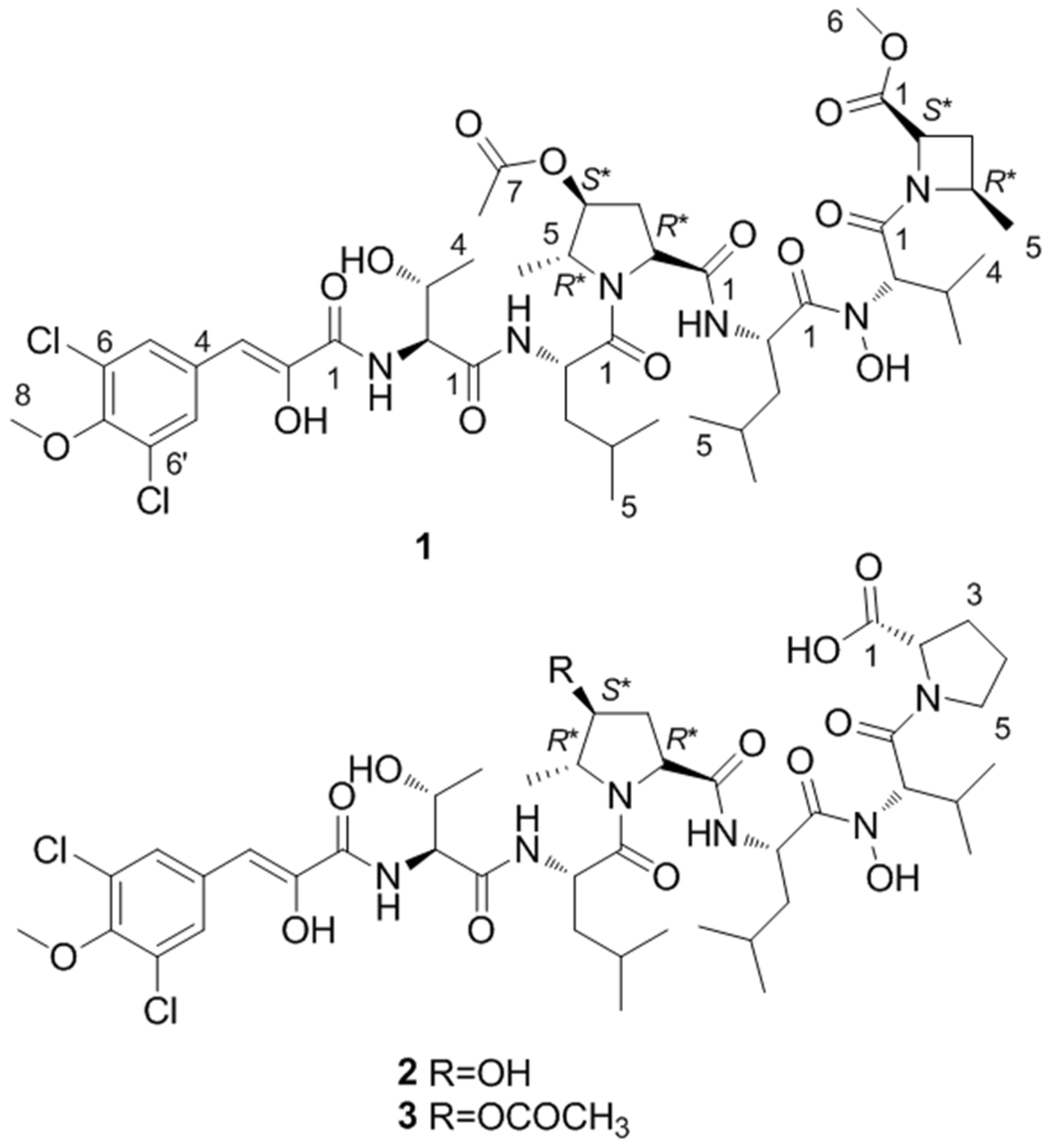
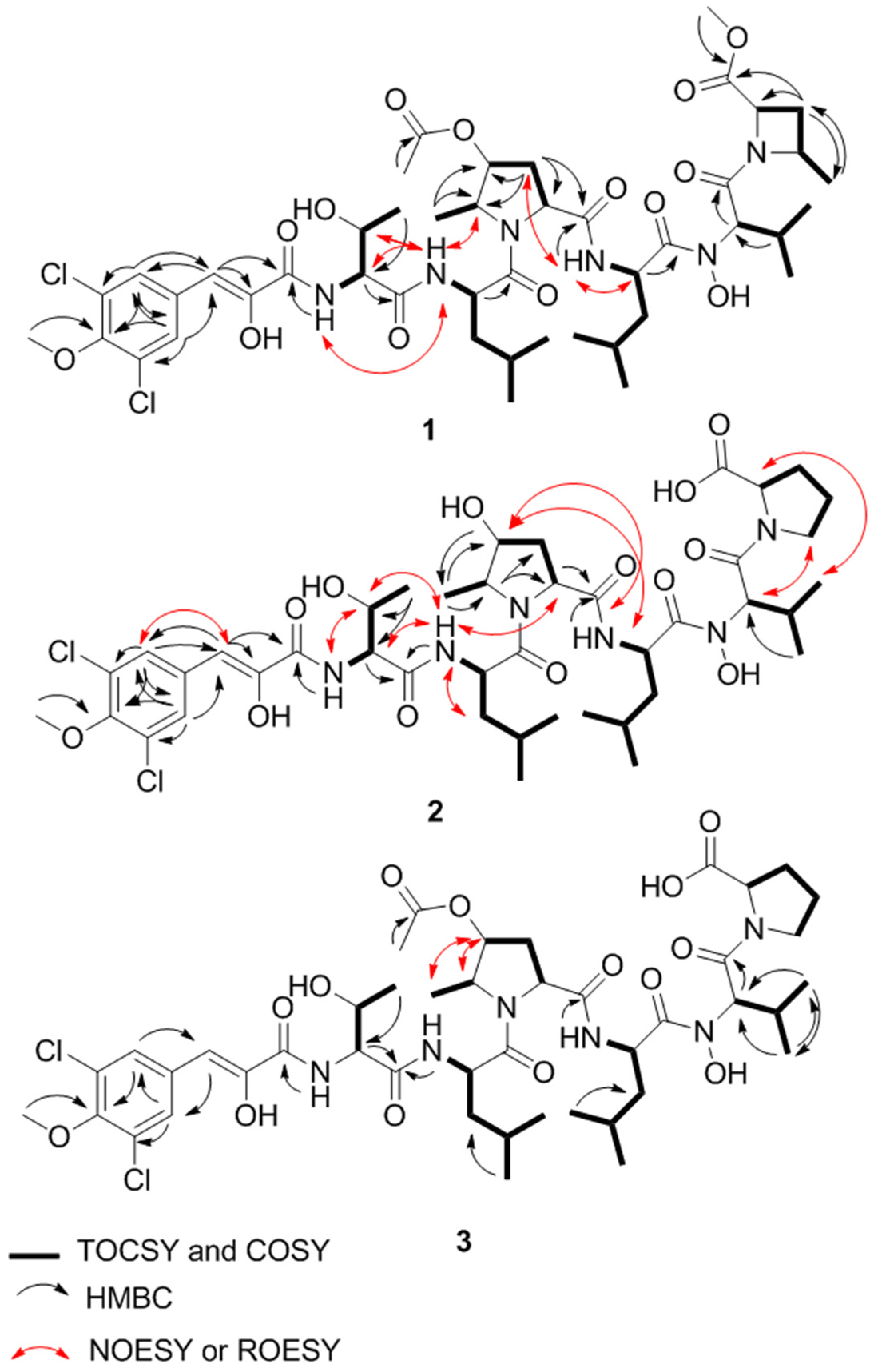


| Residue | Position | Bonnevillamide A (1) | Bonnevillamide B (2) | Bonnevillamide C (3) | |||
|---|---|---|---|---|---|---|---|
| δC a | δH (J in Hz) b | δC a | δH (J in Hz) b | δC c | δH (J in Hz) b | ||
| BVA | 1 | 163.1 C | - | 163.1 C | - | 163.1 C | - |
| 2 | 149.5 C | - | 149.5 C | - | 149.5 C | - | |
| 3 | 115.9 CH | 6.66, s | 116.1 CH | 6.66, s | 115.9 CH | 6.67, s | |
| 4 | 127.0 C | - | 126.9 C | - | d | - | |
| 5/5′ | 129.8 CH | 7.73, s | 129.8 CH | 7.70, s | 129.7 CH | 7.73, s | |
| 6/6′ | 122.7 C | - | 122.7 C | - | 122.6 C | - | |
| 7 | 149.4 C | - | 149.5 C | - | 149.5 C | - | |
| 8 | 60.1 CH3 | 3.67, s | 59.5 CH3 | 3.65, s | 59.4 CH3 | 3.67, s | |
| Thr | 1 | 170.2 C | - | 169.8 C | - | 170.2 C | - |
| 2 | 59.1 CH | 4.26, dd (4.8, 8.6) | 58.8 CH | 4.30, m | 59.1 CH | 4.27, dd (5.0, 8.6) | |
| 3 | 67.2 CH | 3.93, m | 67.5 CH | 3.97, m | 67.1 CH | 3.97, m | |
| 4 | 20.8 CH3 | 1.05, d (6.4) | 20.1 CH3 | 1.02, d (5.2) | 20.7 CH3 | 1.05, d (6.3) | |
| NH | - | 7.67, d (8.6) | - | 7.65, d (7.4) | - | 7.68, d (8.5) | |
| Leu 1 | 1 | 173.8 C | - | 173.7 C | - | d | - |
| 2 | 48.0 CH | 4.98, m | 47.9 CH | 4.95, m | 48.6 CH | 4.49, m | |
| 3 | 40.1 CH2 | 1.37, m; 1.52, m | 40.5 CH2 | 1.34, m; 1.46, m | 41.4 CH2 | 1.31, m; 1.62, m | |
| 4 | 24.4 CH | 1.66, m | 24.7 CH | 1.64, m | 24.7 CH | 1.62, m | |
| 5 | 22.1 CH3 | 0.83, overlap | 23.6 CH3 | 0.85, overlap | 21.5 CH3 | 0.82, overlap | |
| 6 | 22.4 CH3 | 0.83, overlap | 22.2 CH3 | 0.83, overlap | 23.7 CH3 | 0.89, d (6.9) | |
| NH | - | 7.83, d (8.4) | 7.76, d (6.8) | - | 8.26, d (8.0) | ||
| HMPro | 1 | 170.7 C | - | 171.3 C | - | 170.7 C | - |
| 2 | 58.4 CH | 4.43, overlap | 58.8 CH | 4.44, m | 58.4 CH | 4.51, overlap | |
| 3 | 32.1 CH2 | 2.15, m | 35.2 CH2 | 1.92, m; 1.98, m | 32.2 CH2 | 2.16, m; 2.22 m | |
| 4 | 78.5 CH | 4.88, m | 75.3 CH | 3.90, brs | 78.5 CH | 4.89, d | |
| 5 | 60.1 CH | 4.49, m | 62.4 CH | 4.01, m | 60.1 CH | 4.49, overlap | |
| 6 | 19.1 CH3 | 1.18, d (6.8) | 19.3 CH3 | 1.10, d (6.7) | 19.1 CH3 | 1.18, d (6.9) | |
| 7 | 170.5 C | - | - | - | 170.5 C | - | |
| 8 | 21.3 CH3 | 1.99, s | - | - | 21.4 CH3 | 2.00, s | |
| Leu 2 | 1 | 171.2 C | - | 167.3 C | - | d | - |
| 2 | 48.6 CH | 4.49, m | 48.3 CH | 4.66, m | 48.1 CH | 4.97, m | |
| 3 | 41.3 CH2 | 1.31, m; 1.62, m | 41.9 CH2 | 1.38, m; 1.47, m | 40.3 CH2 | 1.37, m; 1.50, m | |
| 4 | 24.7 CH | 1.62, m | 24.5 CH | 1.57, m | 24.5 CH | 1.67, m | |
| 5 | 21.5 CH3 | 0.82, overlap | 23.8 CH3 | 0.84, overlap | 22.1 CH3 | 0.83, overlap | |
| 6 | 23.7 CH3 | 0.88, d (6.9) | 21.5 CH3 | 0.80, overlap | 22.3 CH3 | 0.83, overlap | |
| NH | - | 8.25, d (8.0) | - | 8.20, d (7.6) | - | 7.82, d (8.6) | |
| N-OH-Val | 1 | 168.3 C | - | 167.3 C | - | 167.3 C | - |
| 2 | 61.2 CH | 4.43, m | 62.4 CH | 4.66, m | 62.4 CH | 4.70, d (10.5) | |
| 3 | 26.1 CH2 | 2.31, m | 26.8 CH | 2.30, m | 26.9 CH2 | 2.34, m | |
| 4 | 18.9 CH3 | 0.77, d (6.7) | 19.0 CH3 | 0.76, d (7.0) | 19.0 CH3 | 0.79, d (6.7) | |
| 5 | 19.7 CH3 | 0.84, overlap | 19.5 CH3 | 0.83, overlap | 19.5 CH3 | 0.88, d (6.6) | |
| MACME/Pro | 1 | 171.6 C | - | 171.1 C | - | d | - |
| 2 | 56.2 CH | 4.49, m | 59.1 CH | 4.18, m | 59.2 CH | 4.21, dd (4.6, 8.7) | |
| 3 | 27.9 CH2 | 2.69, m; 1.72, m | 29.2 CH2 | 1.78, m; 2.10, m | 29.2 CH2 | 1.83, m; 2.14, m | |
| 4 | 57.3 CH | 4.36, m | 25.0 CH2 | 1.80, m; 1.90, m | 24.9 CH2 | 1.91, m; 1.83, m | |
| 5 | 22.4 CH3 | 1.47, d (6.2) | 46.9 CH2 | 3.47, m | 46.9 CH2 | 3.50, m | |
| 6 | 52.4 CH3 | 3.67, s | - | - | - | - | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Nielson, J.R.; Peterson, R.T.; Winter, J.M. Bonnevillamides, Linear Heptapeptides Isolated from a Great Salt Lake-Derived Streptomyces sp. Mar. Drugs 2017, 15, 195. https://doi.org/10.3390/md15070195
Wu G, Nielson JR, Peterson RT, Winter JM. Bonnevillamides, Linear Heptapeptides Isolated from a Great Salt Lake-Derived Streptomyces sp. Marine Drugs. 2017; 15(7):195. https://doi.org/10.3390/md15070195
Chicago/Turabian StyleWu, Guangwei, Jason R. Nielson, Randall T. Peterson, and Jaclyn M. Winter. 2017. "Bonnevillamides, Linear Heptapeptides Isolated from a Great Salt Lake-Derived Streptomyces sp." Marine Drugs 15, no. 7: 195. https://doi.org/10.3390/md15070195