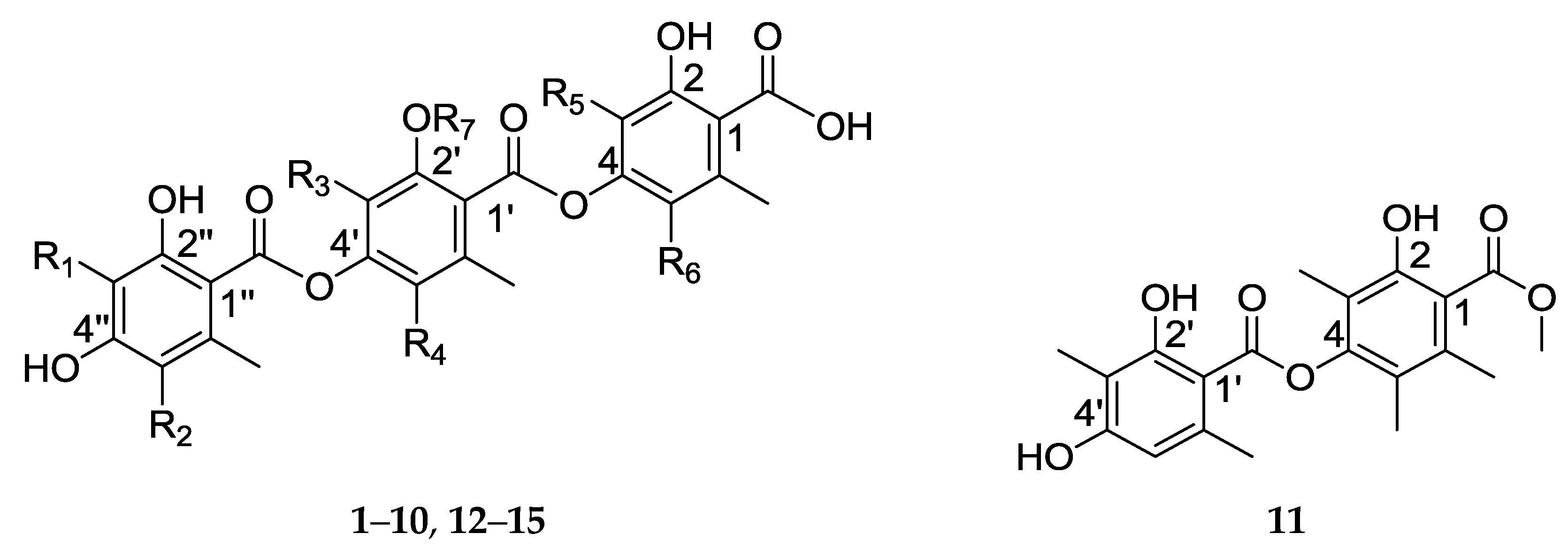
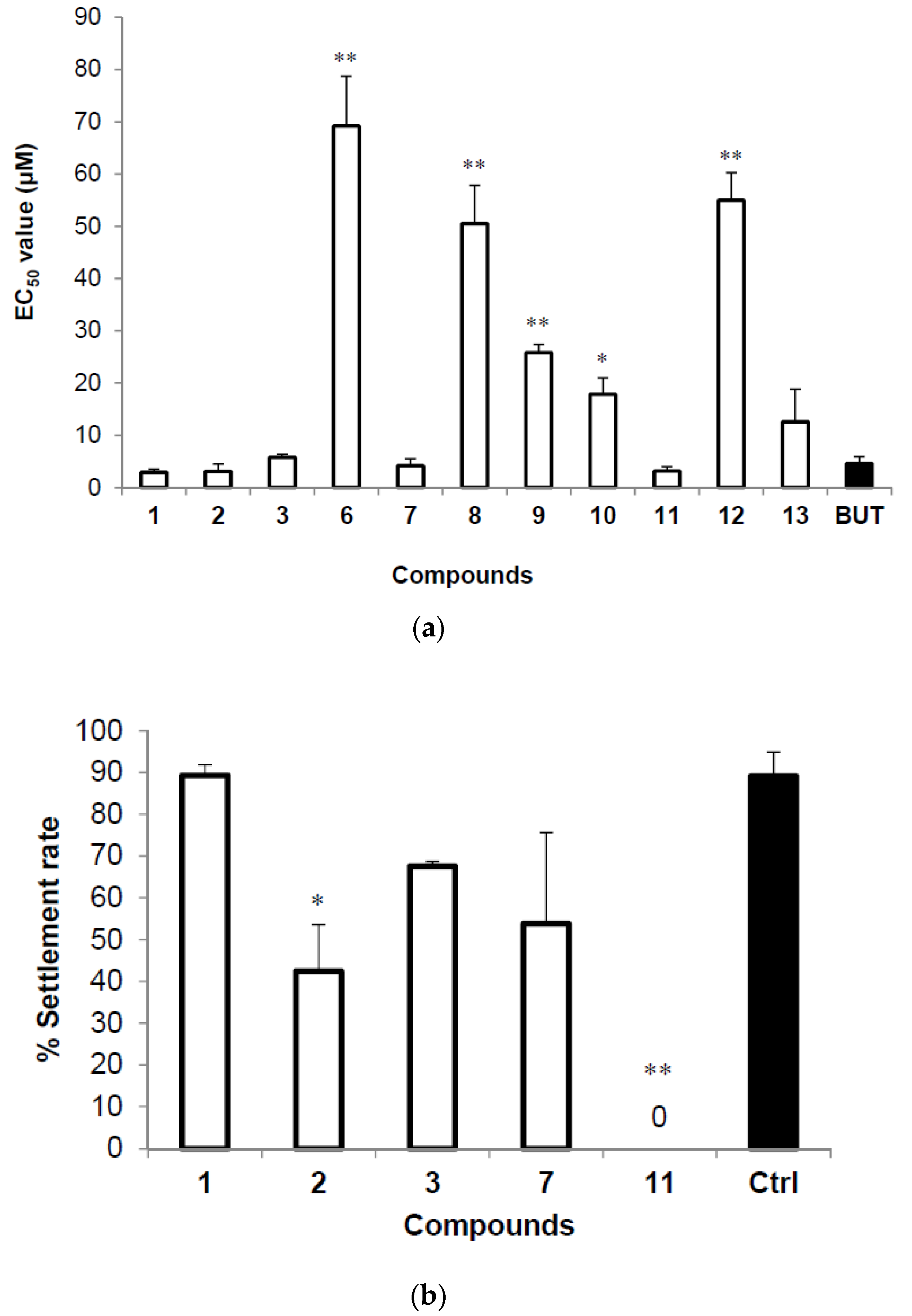
2.2. Structure Elucidation
Fifteen compounds were isolated from the ethyl acetate extract of Thielavia sp. UST030930-004, including the 11 new compounds—thielavins W–Z (1–4) and thielavins Z1–Z7 (5–11)—together with 4 known compounds—thielavins A, H, J, and K (12–15). Their chemical structures were determined using MS, 1D- and 2D-NMR spectroscopy.
Compound
1 was isolated as a white amorphous powder. The positive HRESIMS provided [M + H]
+ at
m/
z 511.1604, corresponding to a molecular formula of C
27H
26O
10 (calcd. for C
27H
27O
10 [M + H]
+ 511.1599), and the UV spectrum revealed absorption at
λmax 218.1, 267.8, and 304.2 nm. The
1H,
13C-NMR (
Table 1) and HSQC spectra of
1 indicated the presence of 6 methyl groups at
δH 2.52 (3H, s), 2.40 (3H, s), 2.29 (3H, s), 2.06 (3H, s), 2.06 (3H, s), 1.99 (3H, s), 18 olefinic carbons, including fifteen quaternary carbons at
δC 138.7, 165.4, 114.2, 149.5, 114.7, 131.8, 150.7, 116.5, 149.1, 120.8, 121.6, 141.0, 161.4, 160.8, and 107.6, and 3 tertiary carbons at
δC 110.3, 100.6, 110.3. Three carbonyl signals were also observed at
δC 172.0, 167.1, and 166.7. These data suggested that the compound is similar to thielavin H (13) [
9]. The only difference between
1 and thielavin H was that one methyl group in thielavin H was absent in
1. The structure of
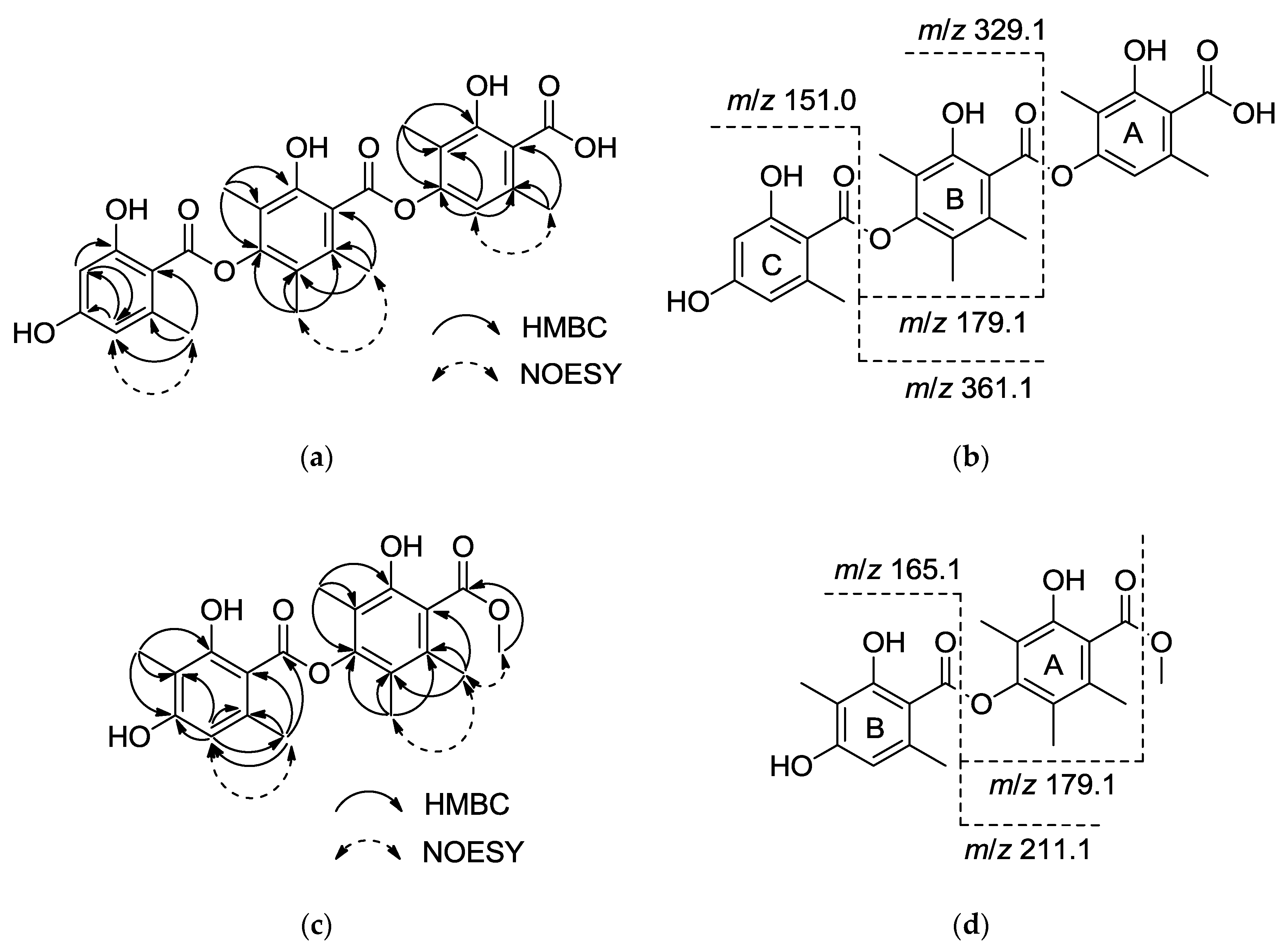
1 was established based on HMBC correlations and in-source collision-induced dissociation (ISCID) fragment ions. The HMBC correlations observed from the methyl group
δH 1.99 (3-Me) to
δC 165.4 (C-2), 114.2 (C-3) and 149.5 (C-4), from
δH 2.52 (6-Me) to
δC 138.7 (C-1), 110.3 (C-5) and 114.7 (C-6), and from an olefinic proton
δH 6.18 (H-5) to C-4 and C-6 indicated the presence of a 2,4-dioxygenated-3,6-dimethylbenzene substructure (A). The second substructure (B) was confirmed by long-range couplings from
δH 2.06 (3′-Me) to
δC 150.7 (C-2′), 116.5 (C-3′) and 149.1(C-4′), and from
δH 2.06 (5′-Me) to
δC 120.8 (C-5′), 121.6 (C-6′) and C-4′, and couplings from another methyl group
δH 2.29 (6′-Me) to
δC 131.8 (C-1′), C-5′ and C-6′. The HMBC correlations from
δH 2.40 (6″-Me) to
δC 141.0 (C-1″), 110.3 (C-5″) and 107.6 (C-6″), correlations from an olefinic proton
δH 6.26 (H-3″) to
δC 161.4 (C-2″), C-5″, and correlations from olefinic proton
δH 6.26 (H-5″) to
δC 100.6 (C-3″), 160.8 (C-4″) and C-6″, indicated the presence of a 2,4-dioxygenated-6-methylbenzene substructure (C). From the deduction above, the remaining three carbonyl groups can only be assigned to C-1, C-1′ and C-1″, and these carbonyl groups may be interchangeable as no HMBC correlations were observed (
Figure 2a). The positive ISCID MS/MS gave ion peaks at
m/
z 329.1, 151.0, 361.1 and 179.1, suggesting the absence of [M − A]
+, [M − A − B]
+, [M − C + 2H]
+ and [M − A − C + 2H]
+ fragments (
Figure 2b), indicating that the order of the substructures is A − B − C (
Figure 2b). Thus the structure of
1 was determined (shown in
Figure 1) and named thielavin W.
Comparison of the UV, NMR, HRESIMS and ISCID MS/MS data shows that compounds
2–
10 have the same core structure as compound
1. Their structures were determined using the procedure described for compound
1, and named thielavins X–Z and Z
1–Z
5, respectively. All of the
1H and
13C-NMR assignments of the new compounds
2–
10 are summarized in
Table 1,
Table 2,
Table 3 and
Table 4.
Compound
11 was obtained as a white amorphous powder. Positive HRESIMS gave [M + H]
+ as
m/
z 375.1464, and the molecular formula was established as C
20H
22O
7 (calcd. for C
20H
23O
7 [M + H]
+ 375.1438). The maximum UV absorption occurred at
λmax 215.8, 276.1, and 308.9 nm, and the
1H NMR (
Table 3) revealed the presence of 5 quaternary methyl signals [
δH 1.96 (3H, s), 1.96 (3H, s), 2.13 (3H, s), 1.97 (3H, s), and 2.54 (3H, s)], 1 methoxy signal [
δH 3.83 (3H, s)], and 1 olefinic proton signal [
δH 6.40 (1H, s)]. The
13C-NMR and HSQC results (
Table 3) indicated the presence of 12 olefinic carbons, including 11 quaternary carbons at
δC 162.6, 161.0, 150.7, 148.5, 139.4, 132.1, 121.4, 120.1, 116.0, 108.6, and 102.7, 1 tertiary carbon at
δC 111.0 and 2 carbonyl groups at
δC 168.7 and 168.9. Based on these data, compound
11 was proposed as a thielavin derivative consisting of two hydroxybenzoic acid groups with a methyl ester terminus. HMBC revealed correlations (
Figure 2c) between
δH 1.96 (3-Me) and
δC 150.7 (C-2), 116.0 (C-3) and 148.5 (C-4), between
δH 1.96 (s, 5-Me) and
δC 120.1 (C-5), 121.4 (C-6), C-4, and between
δH 2.13 (6-Me) to
δC 132.1 (C-1), C-5 and C-6, and also revealed long range correlations between
δH 3.83 (1-COO
Me) and
δC 168.7 (1-C=O). NOESY results show the correlation from protons 6-Me to methoxy proton (1-COO
Me), indicating the presence of a 2-hydroxyl-4-oxygenated-3,5,6-trimethylbenzoic methyl ester subunit, corresponding to the ion fragment of
m/
z 179.1. The HMBC correlations (
Figure 2c) from
δH 1.97 (3′-Me) to
δC 162.6 (C-2′), 108.6 (C-3′) and 161.0 (C-4′), from
δH 6.40 (H-5′) to
δC 102.7 (C-6′), 24.0 (6′-Me), C-3′ and C-4′, and from
δH 2.54 (6′-Me) to
δC 139.4 (C-1′), 111.0 (C-5′) and C-6′, suggested the presence of a 2-hydroxyl-4-oxygenated-3,6-dimethylbenzoyl subunit, corresponding to an ion fragment with
m/
z 211.1 (
Figure 2d). These two units should be connected by an ester bond, but there was no correlation between the two units and the connection was only supported by ion fragments [M − B + 2H]
+, [M – Ome − B + H]
+ and [M – Ome − A]
+ at
m/
z 211.1, 179.1 and 165.1, respectively. Thus, the structure of 11 was determined (shown in
Figure 1) and named thielavin Z
7.
Compounds
12–
15 were identified as thielavin A (
12), thielavin H (
13), thielavin J (
14), and thielavin K (
15), by comparison of their spectral data with those reported in the literature [
9,
10].