Identification of 2-keto-3-deoxy-d-Gluconate Kinase and 2-keto-3-deoxy-d-Phosphogluconate Aldolase in an Alginate-Assimilating Bacterium, Flavobacterium sp. Strain UMI-01
Abstract
:1. Introduction
2. Results
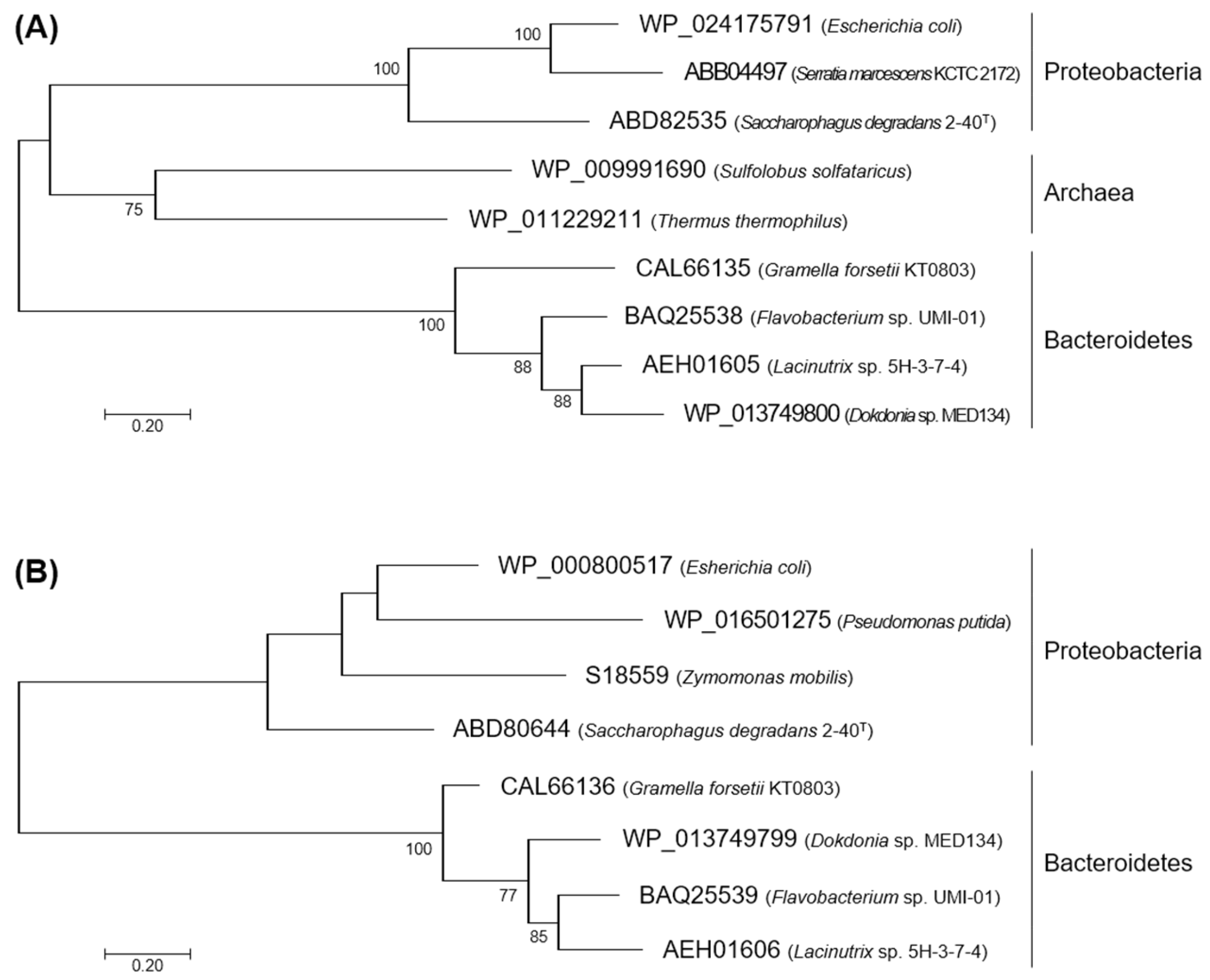
2.1. Characteristics in the Primary Structures of FlKin and FlAld
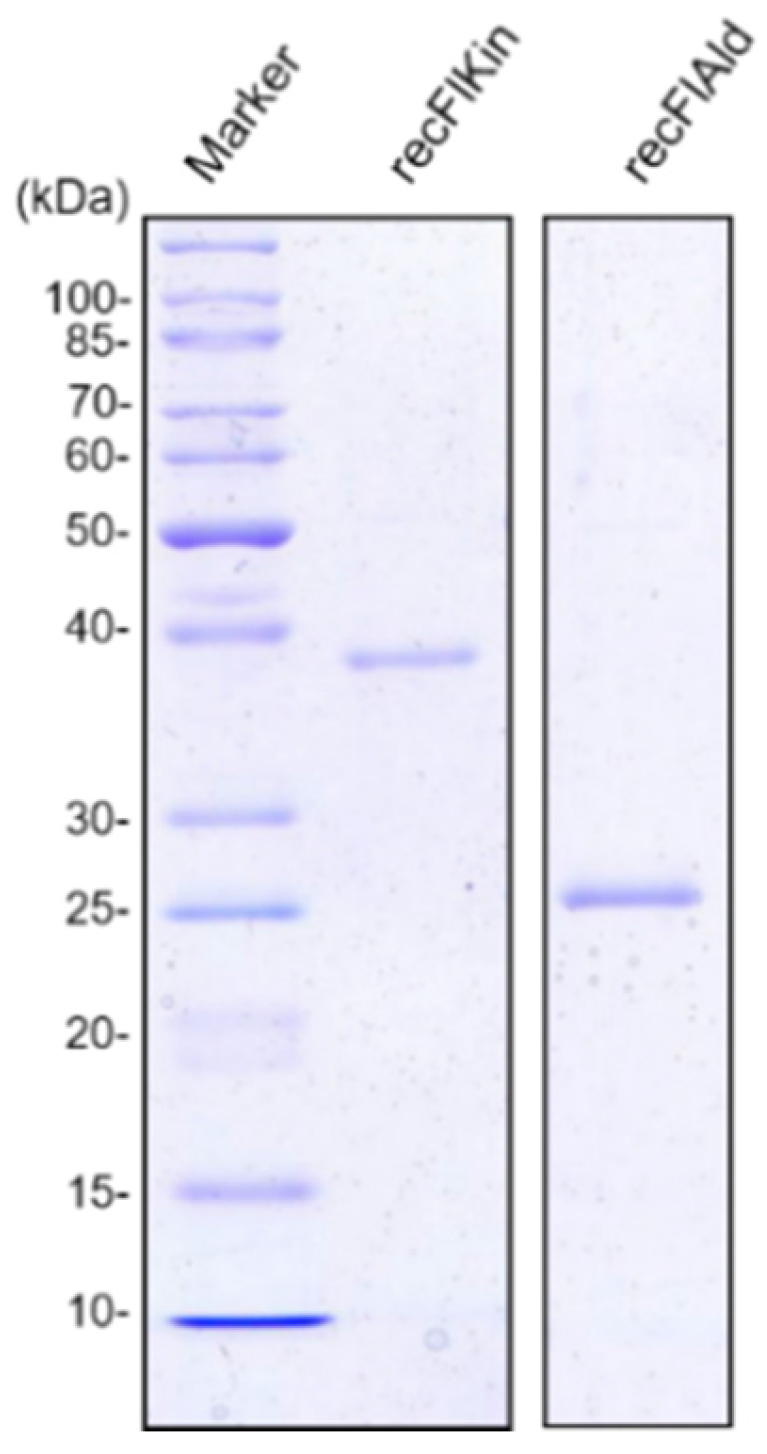
2.2. Production of recFlKin and recFlAld, and Their Reaction Products
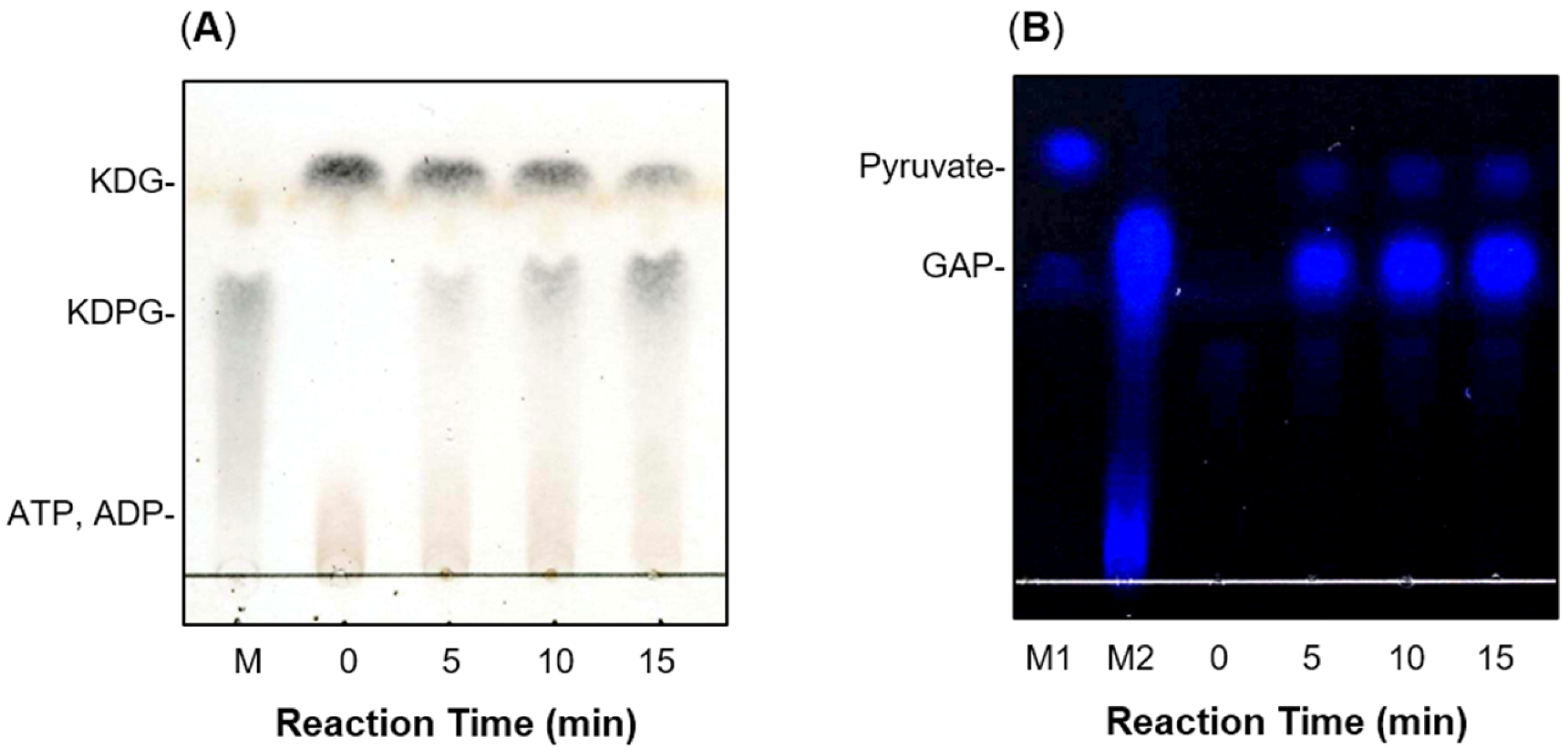
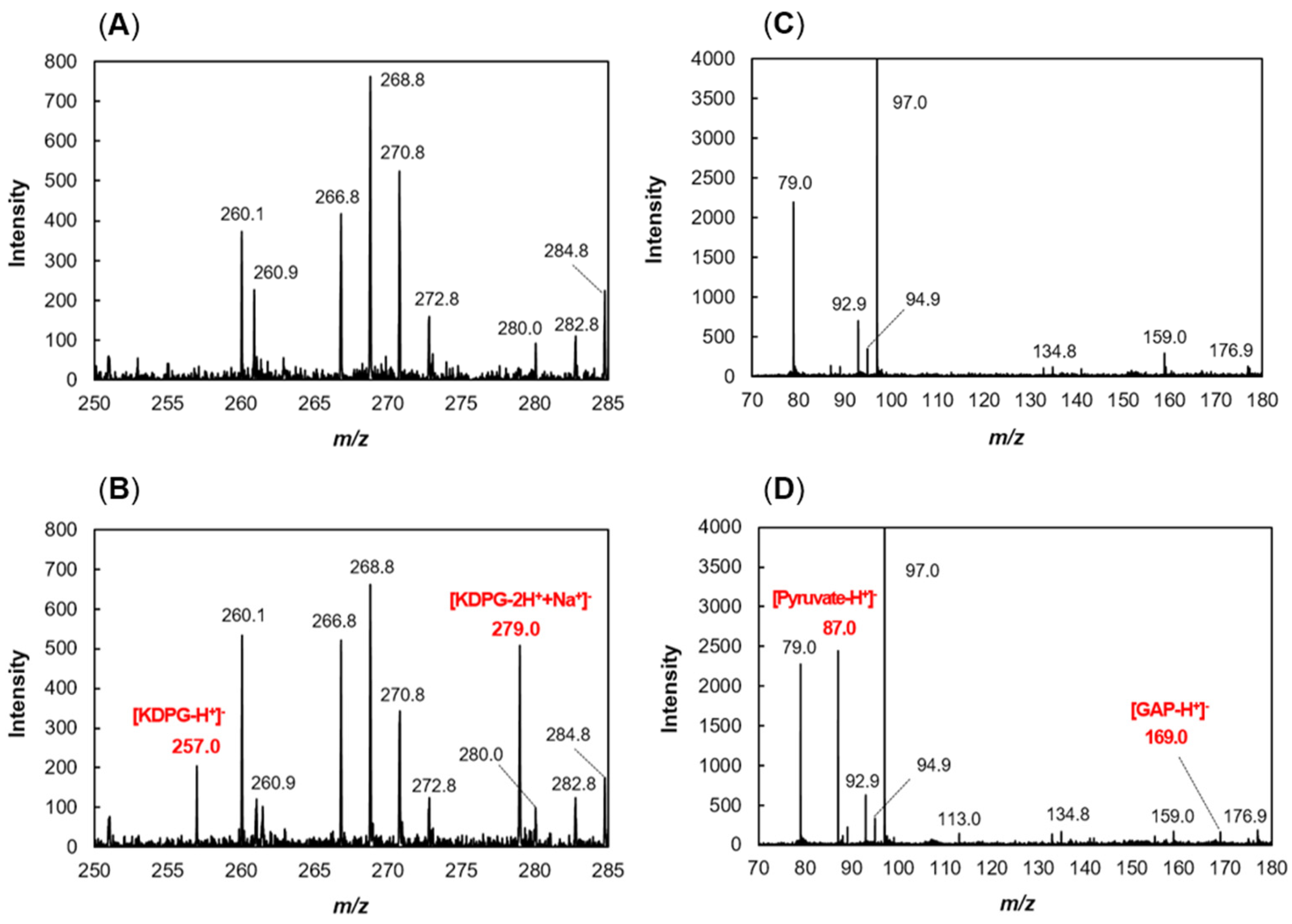
2.3. Enzymatic Properties of recFlKin and recFlAld
2.4. Construction of In Vitro Alginate-Metabolizing System Using Recombinant Enzymes
3. Discussion
3.1. Alginate-Metabolizing Enzymes of Flavobacterium sp. Strain UMI-01
3.2. Properties of recFlKin and recFlAld
3.3. Construction of In Vitro Alginate-Metabolizing System
3.4. Production of a High-Value Product KDPG from Alginate
4. Experimental Section
4.1. Materials
4.2. Phylogenetic Analysis for KDG Kinases and KDPG Aldolases
4.3. Cloning, Expression, and Purification of Recombinant FlKin and FlAld
4.4. Preparation of KDG
4.5. Preparation of KDPG
4.6. Assay for KDPG Aldolase Activity
4.7. Assay for KDG Kinase Activity
4.8. Construction of In Vitro Alginate-Metabolizing System from Recombinant Enzymes
4.9. Determination of Unsaturated Sugars
4.10. Thin-Layer Chromatography
4.11. Mass Spectrometry
4.12. SDS-PAGE
4.13. Determination of Protein Concentration
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haug, A.; Larsen, B.; Smidsrod, O. Studies on the sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 1967, 21, 691–704. [Google Scholar] [CrossRef]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. Alginate lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, Y.; Umemura, K.; Adachi, T. Promotion of barley root elongation under hypoxic conditions by alginate lyase-lysate (A.L.L.). Biosci. Biotechnol. Biochem. 1994, 58, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Iwamoto, Y.; Kitamura, Y.; Oda, T.; Muramatsu, T. Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci. Biotechnol. Biochem. 2003, 67, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Endo, T.; Nakakita, R.; Murata, K.; Yonemoto, Y.; Okayama, K. Effect of depolymerized alginates on the growth of bifidobacteria. Biosci. Biotechnol. Biochem. 1992, 56, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Ariyo, B.; Tamerler, C.; Bucke, C.; Keshavarz, T. Enhanced penicillin production by oligosaccharides from batch cultures of Penicillium chrysogenum in stirred-tank reactors. FEMS Microbiol. Lett. 1998, 166, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Neubert, R.H.H. Screening for new antioxidative compounds for topical administration using skin lipid model systems. J. Pharm. Pharm. Sci. 2005, 8, 494–506. [Google Scholar] [PubMed]
- Khodagholi, F.; Eftekharzadeh, B.; Yazdanparast, R. A new artificial chaperone for protein refolding: Sequential use of detergent and alginate. Protein J. 2008, 27, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.-J.; Son, E.-W.; Rhee, D.-K.; Pyo, S. Modulation of tnf-α-induced icam-1 expression, no and h202 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch. Pharm. Res. 2003, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- An, Q.D.; Zhang, G.L.; Wu, H.T.; Zhang, Z.C.; Zheng, G.S.; Luan, L.; Murata, Y.; Li, X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 2009, 106, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Enquist-Newman, M.; Faust, A.M.E.; Bravo, D.D.; Santos, C.N.S.; Raisner, R.M.; Hanel, A.; Sarvahowman, P.; Le, C.; Regitsky, D.D.; Cooper, S.R.; et al. Efficient ethanol production from brown macroalgae sugars by a synthetic yeast platform. Nature 2014, 505, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Yoneyama, F.; Kawai, S.; Hashimoto, W.; Murata, K. Bioethanol production from marine biomass alginate by metabolically engineered bacteria. Energy Environ. Sci. 2011, 4, 2575. [Google Scholar] [CrossRef]
- Miyake, H.; Yamaki, T.; Nakamura, T.; Ishibashi, H.; Fukuiri, Y.; Sakuma, A.; Komatsu, H.; Anso, T.; Togashi, K.; Umetani, H. Gluconate dehydratase. U.S. Patent 7,125,704 B2, 24 October 2006. [Google Scholar]
- Yoon, H.J.; Hashimoto, W.; Miyake, O.; Okamoto, M.; Mikami, B.; Murata, K. Overexpression in Escherichia coli, purification, and characterization of Sphingomonas sp. A1 alginate lyases. Protein Expr. Purif. 2000, 19, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, W.; Miyake, O.; Momma, K.; Kawai, S.; Murata, K. Molecular identification of oligoalginate lyase of Sphingomonas sp. strain A1 as one of the enzymes required for complete depolymerization of alginate. J. Bacteriol. 2000, 182, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Takase, R.; Ochiai, A.; Mikami, B.; Hashimoto, W.; Murata, K. Molecular identification of unsaturated uronate reductase prerequisite for alginate metabolism in Sphingomonas sp. A1. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Takase, R.; Mikami, B.; Kawai, S.; Murata, K.; Hashimoto, W. Structure-based conversion of the coenzyme requirement of a short-chain dehydrogenase/reductase involved in bacterial alginate metabolism. J. Biol. Chem. 2014, 289, 33198–33214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preiss, J.; Ashwell, G. Alginic acid metabolism in bacteria I. J. Biol. Chem. 1962, 237, 309–316. [Google Scholar] [PubMed]
- Preiss, J.; Ashwell, G. Alginic acid metabolism in bacteria II. J. Biol. Chem. 1962, 237, 317–321. [Google Scholar] [PubMed]
- Kim, H.T.; Ko, H.J.; Kim, N.; Kim, D.; Lee, D.; Choi, I.G.; Woo, H.C.; Kim, M.D.; Kim, K.H. Characterization of a recombinant endo-type alginate lyase (Alg7D) from Saccharophagus degradans. Biotechnol. Lett. 2012, 34, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Chung, J.H.; Wang, D.; Lee, J.; Woo, H.C.; Choi, I.G.; Kim, K.H. Depolymerization of alginate into a monomeric sugar acid using Alg17C, an exo-oligoalginate lyase cloned from Saccharophagus degradans 2-40. Appl. Microbiol. Biotechnol. 2012, 93, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Morisaka, H.; Aburaya, S.; Tatsukami, Y.; Kuroda, K.; Ueda, M. Putative alginate assimilation process of the marine bacterium Saccharophagus degradans 2-40 based on quantitative proteomic analysis. Mar. Biotechnol. 2016, 18, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Wang, D.; Yun, E.J.; Kim, S.; Kim, S.R.; Kim, K.H. Validation of the metabolic pathway of the alginate-derived monomer in Saccharophagus degradans 2-40T by gas chromatography–mass spectrometry. Process Biochem. 2016, 51, 1374–1379. [Google Scholar] [CrossRef]
- Inoue, A.; Takadono, K.; Nishiyama, R.; Tajima, K.; Kobayashi, T.; Ojima, T. Characterization of an alginate lyase, FlAlyA, from Flavobacterium sp. strain UMI-01 and its expression in Escherichia coli. Mar. Drugs 2014, 12, 4693–4712. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Nishiyama, R.; Ojima, T. The alginate lyases FlAlyA, FlAlyB, FlAlyC, and FlAlex from Flavobacterium sp. UMI-01 have distinct roles in the complete degradation of alginate. Algal Res. 2016, 19, 355–362. [Google Scholar] [CrossRef]
- Inoue, A.; Nishiyama, R.; Mochizuki, S.; Ojima, T. Identification of a 4-deoxy-l-erythro-5-hexoseulose uronic acid reductase, FlRed, in an alginolytic bacterium Flavobacterium sp. strain UMI-01. Mar. Drugs 2015, 13, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Badur, A.H.; Jagtap, S.S.; Yalamanchili, G.; Lee, J.-K.; Zhao, H.; Rao, C.V. Alginate lyases from alginate-degrading Vibrio splendidus 12B01 are endolytic. Appl. Environ. Microbiol. 2015, 81, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Ojima, T.; Nishita, K. cDNA cloning of an alginate lyase from abalone, Haliotis discus hannai. Carbohydr. Res. 2003, 338, 2841–2852. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Suzuki, K.I.; Inoue, A.; Ojima, T. A novel oligoalginate lyase from abalone, Haliotis discus hannai, that releases disaccharide from alginate polymer in an exolytic manner. Carbohydr. Res. 2006, 341, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Inoue, A.; Tanaka, H.; Ojima, T. Isolation and characterization of two alginate lyase isozymes, AkAly28 and AkAly33, from the common sea hare Aplysia kurodai. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Inoue, A.; Tanaka, H.; Ojima, T. cDNA cloning of an alginate lyase from a marine gastropod Aplysia kurodai and assessment of catalutically important residues of this enzyme. Biochimie 2011, 93, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Mashino, C.; Uji, T.; Saga, N.; Mikami, K.; Olima, T. Characterization of an eukaryotic PL-7 alginate lyase in the marine red alga Pyropia yezoensis. Curr. Biotechnol. 2015, 4, 240–248. [Google Scholar] [CrossRef]
- Gacesa, P. Enzymic degradation of alginates. Int. J. Biochem. 1992, 24, 545–552. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Lee, S.M.; Robb, M.; Hof, F.; Bar, C.; Abe, K.T.; Hehemann, J.H.; McLean, R.; Abbott, D.W.; Boraston, A.B. KdgF, the missing link in the microbial metabolism of uronate sugars from pectin and alginate. Proc. Natl. Acad. Sci. USA 2016, 113, 6188–6193. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Nishiyama, R.; Inoue, A.; Ojima, T. A novel aldo-keto reductase HdRed from the pacific abalone Haliotis discus hannai, which reduces alginate-derived 4-deoxy-l-erythro-5-hexoseulose uronic acid to 2-keto-3-deoxy-d-gluconate. J. Biol. Chem. 2015, 290, 30962–30974. [Google Scholar] [CrossRef] [PubMed]
- Cynkin, M.A.; Ashwell, G. Uronic acid metabolism in bacteria. J. Biol. Chem. 1960, 235, 1576–1579. [Google Scholar] [PubMed]
- Lee, Y.S.; Park, I.H.; Yoo, J.S.; Kim, H.S.; Chung, S.Y.; Chandra, M.R.G.; Choi, Y.L. Gene expression and characterization of 2-keto-3-deoxy-gluconate kinase, a key enzyme in the modified Entner-Doudoroff pathway of Serratia marcescens KCTC 2172. Electron. J. Biotechnol. 2009, 12. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.B. Characterization of Sulfolobus solfataricus 2-keto-3-deoxy-d-gluconate kinase in the modified Entner-Doudoroff pathway. Biosci. Biotechnol. Biochem. 2006, 70, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Lamble, H.J.; Theodossis, A.; Milburn, C.C.; Taylor, G.L.; Bull, S.D.; Hough, D.W.; Danson, M.J. Promiscuity in the part-phosphorylative Entner-Doudoroff pathway of the archaeon Sulfolobus solfataricus. FEBS Lett. 2005, 579, 6865–6869. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.A.; Kerou, M.; Lamble, H.J.; Bull, S.D.; Hough, D.W.; Danson, M.J.; Taylor, G.L. The structure of Sulfolobus solfataricus 2-keto-3-deoxygluconate kinase. Acta Crystallogr. Sect. D Biol. Crystallogr. 2008, 64, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, N.; Inagaki, E.; Yasuike, K.; Takio, K.; Tahirov, T.H. Structure of Thermus thermophilus 2-keto-3-deoxygluconate kinase: Evidence for recognition of an open chain substrate. J. Mol. Biol. 2004, 340, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Shelton, M.C.; Cotterill, I.C.; Novak, S.T.A.; Poonawala, R.M.; Sudarshan, S.; Toone, E.J. 2-Keto-3-deoxy-6-phosphogluconate aldolases as catalysts for stereocontrolled carbon-carbon bond formation. J. Am. Chem. Soc. 1996, 118, 2117–2125. [Google Scholar] [CrossRef]
- Allard, J.; Grochulski, P.; Sygusch, J. Covalent intermediate trapped in 2-keto-3-deoxy-6-phosphogluconate (KDPG) aldolase structure at 1.95-A resolution. Proc. Natl. Acad. Sci. USA 2001, 98, 3679–3684. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.J.; Watanabe, L.; Lebioda, L.; Arni, R.K. Structure of 2-keto-3-deoxy-6-phosphogluconate (KDPG) aldolase from Pseudomonas putida. Acta Crystallogr. D Biol. Crystallogr. 2003, 59, 1454–1458. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Kawakami, R.; Kanai, Y.; Goda, S.; Sakuraba, H. Gene expression and characterization of 2-keto-3-deoxygluconate kinase, a key enzyme in the modified Entner-Doudoroff pathway of the aerobic and acidophilic hyperthermophile Sulfolobus tokodaii. Protein Expr. Purif. 2007, 54, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Ettema, T.J.G.; Tjaden, B.; Geerling, A.C.M.; Oost, J.V.D.; Siebers, B. The semi-phosphorylative Entner-Doudoroff pathway in hyperthermophilic archaea: A re-evaluation. Biochem. J. 2005, 390, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Reher, M.; Fuhrer, T.; Bott, M.; Schönheit, P. The nonphosphorylative entner-doudoroff pathway in the thermoacidophilic euryarchaeon Picrophilus torridus involves a novel 2-Keto-3-deoxygluconate-specific aldolase. J. Bacteriol. 2010, 192, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, I.C.; Shelton, M.C.; Machemer, D.E.W.; Henderson, D.P.; Toone, E.J. Effect of phosphorylation on the reaction rate of unnatural electrophiles with 2-keto-3-deoxy-6-phosphogluconate aldolase. J. Chem. Soc. Trans. 1998, 1, 1335–1341. [Google Scholar] [CrossRef]
- Kabisch, A.; Otto, A.; Konig, S.; Becher, D.; Albrecht, D.; Schuler, M.; Teeling, H.; Amann, R.I.; Scheweder, T. Functional characterization of polysaccharide utilization loci in the marine Bacteroidetes ‘Gramella forsetii’ KT0803. ISME J. 2014, 8, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Weissbach, A.; Hurwitz, J. The Formation of 2-Keto-3-deoxyheptonie Acid in Extracts of Escherichia coli B. I. Identification. J. Biol. Chem. 1959, 234, 705–710. [Google Scholar] [PubMed]
- Porzio, M.A.; Pearson, A.M. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim. Biophys. Acta (BBA) Protein Struct. 1977, 490, 27–34. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the dolin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]





| Primer Name | Nucleotide Sequence |
|---|---|
| recFlKin-F | 5′-AGGTAATACACCATGAAAAAAGTAGTCACTTTTGG-3′ |
| recFlKin-R | 5′-CACCTCCACCGGATCCTCTTGAAACTTTTCCTGAAA-3′ |
| recFlAld-F | 5′-ATGTAATACACCATGGCTCAATTTTCAAGAATAGA-3′ |
| recFlAld-R | 5′-CACCTCCACCGGATCCTTGTTTTAACTCTTTAATGA-3′ |
| Enzymes | Substrates/Products | Concentration (mM) | Yield (%) |
|---|---|---|---|
| None | Alginate a | 10 a | - |
| recFlAlyA | Oligoalginates | 4.2 ± 0.06 | - |
| recFlAlyA + recFlAlyB + recFlAlex | DEH | 9.8 ± 0.34 | 98 |
| recFlAlyA + recFlAlyB + recFlAlex + recFlRed | KDG | 9.8 ± 1.0 | 98 |
| recFlAlyA + recFlAlyB + recFlAlex + recFlRed + recFlKin | KDPG | 8.1 ± 0.54 | 81 |
| recFlAlyA + recFlAlyB + recFlAlex + recFlRed + recFlKin + recFlAld | Pyruvate (and GAP) | 3.8 ± 0.33 | 38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, R.; Inoue, A.; Ojima, T. Identification of 2-keto-3-deoxy-d-Gluconate Kinase and 2-keto-3-deoxy-d-Phosphogluconate Aldolase in an Alginate-Assimilating Bacterium, Flavobacterium sp. Strain UMI-01. Mar. Drugs 2017, 15, 37. https://doi.org/10.3390/md15020037
Nishiyama R, Inoue A, Ojima T. Identification of 2-keto-3-deoxy-d-Gluconate Kinase and 2-keto-3-deoxy-d-Phosphogluconate Aldolase in an Alginate-Assimilating Bacterium, Flavobacterium sp. Strain UMI-01. Marine Drugs. 2017; 15(2):37. https://doi.org/10.3390/md15020037
Chicago/Turabian StyleNishiyama, Ryuji, Akira Inoue, and Takao Ojima. 2017. "Identification of 2-keto-3-deoxy-d-Gluconate Kinase and 2-keto-3-deoxy-d-Phosphogluconate Aldolase in an Alginate-Assimilating Bacterium, Flavobacterium sp. Strain UMI-01" Marine Drugs 15, no. 2: 37. https://doi.org/10.3390/md15020037





