Structure-Based Design and Synthesis of a New Phenylboronic-Modified Affinity Medium for Metalloprotease Purification
Abstract
:1. Introduction
2. Results and Discussion
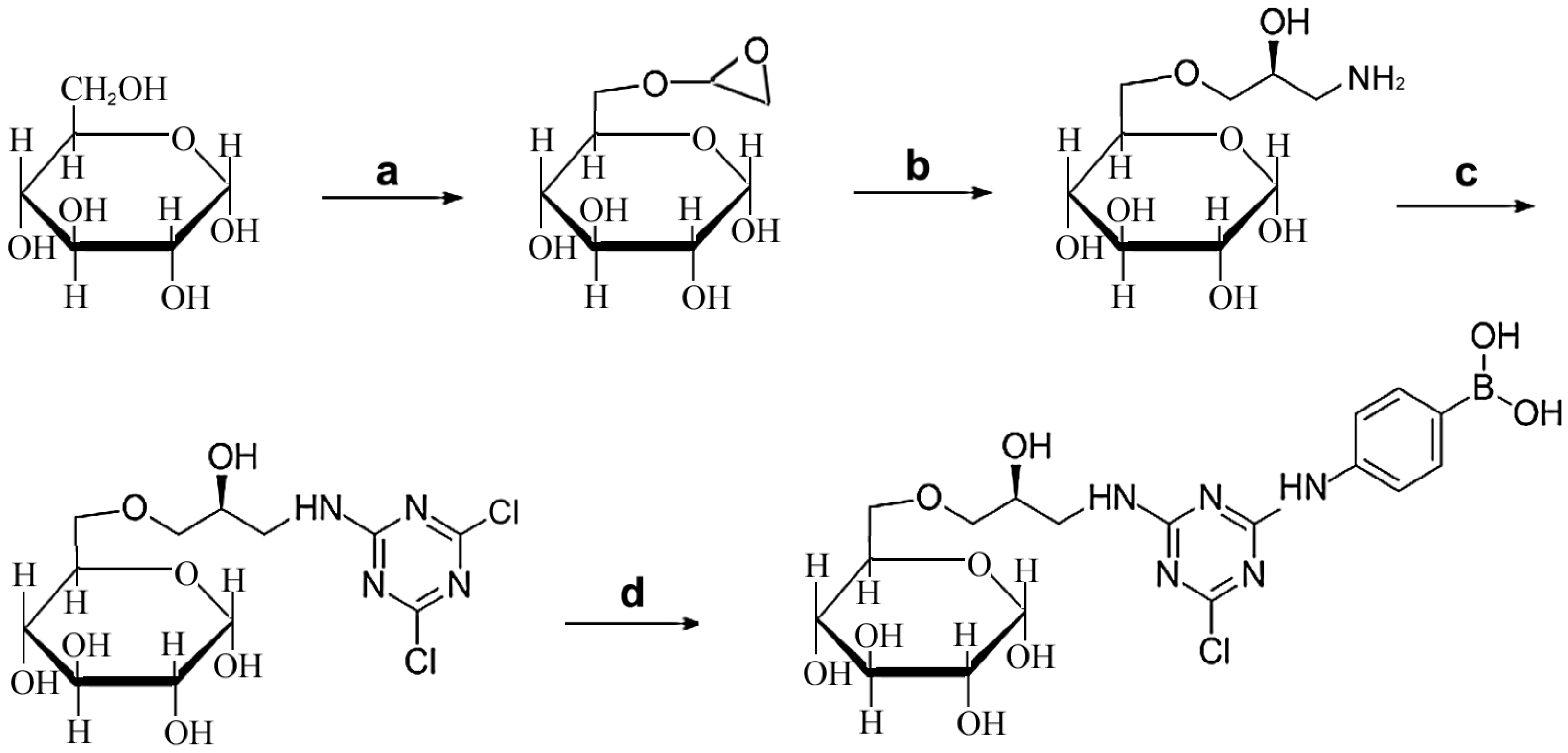
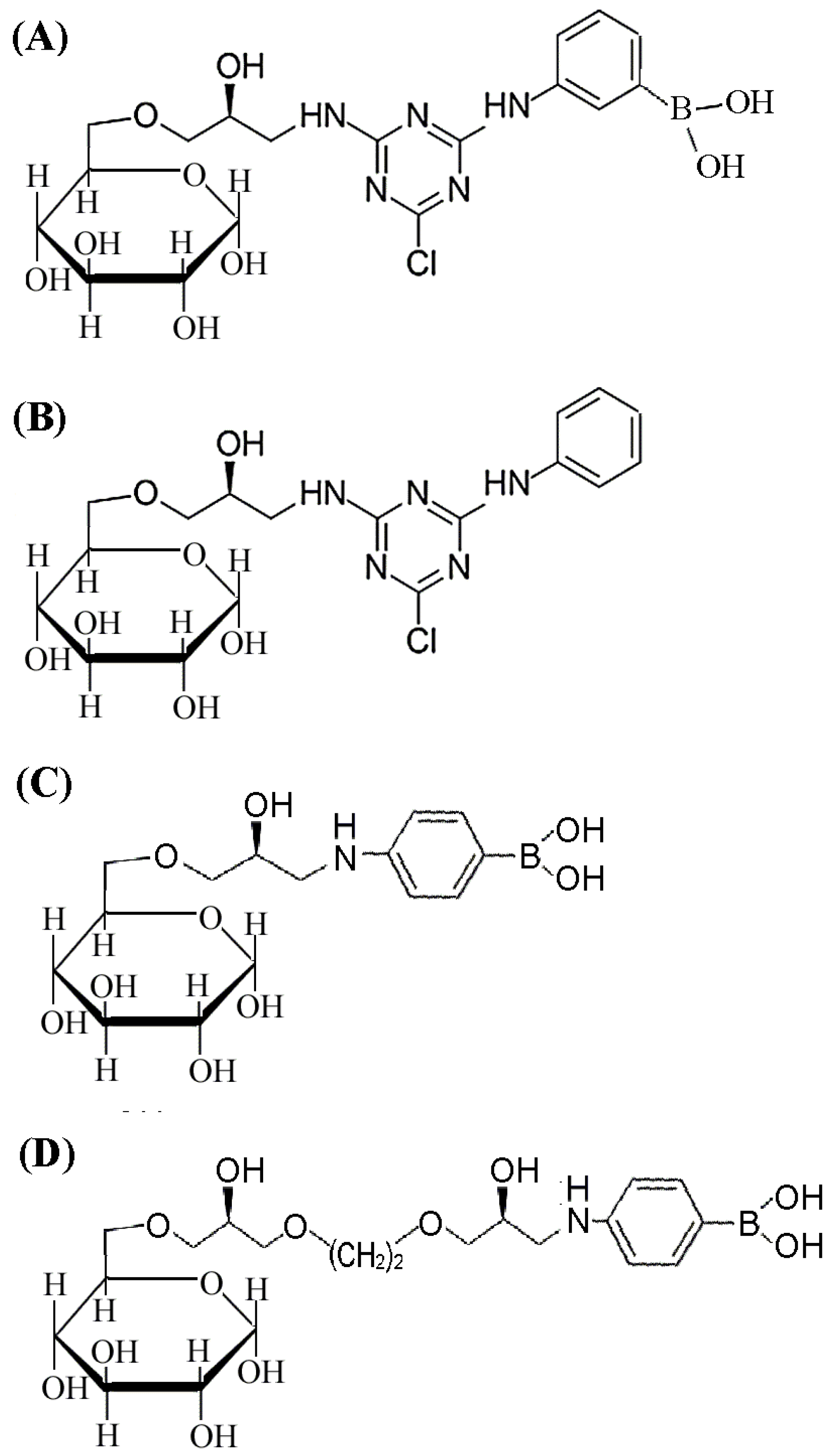
2.1. Design and Synthesis of Affinity Medium for Metalloprotease Purification
2.2. Binding Analysis for 4-APBA-Modified Medium and MP
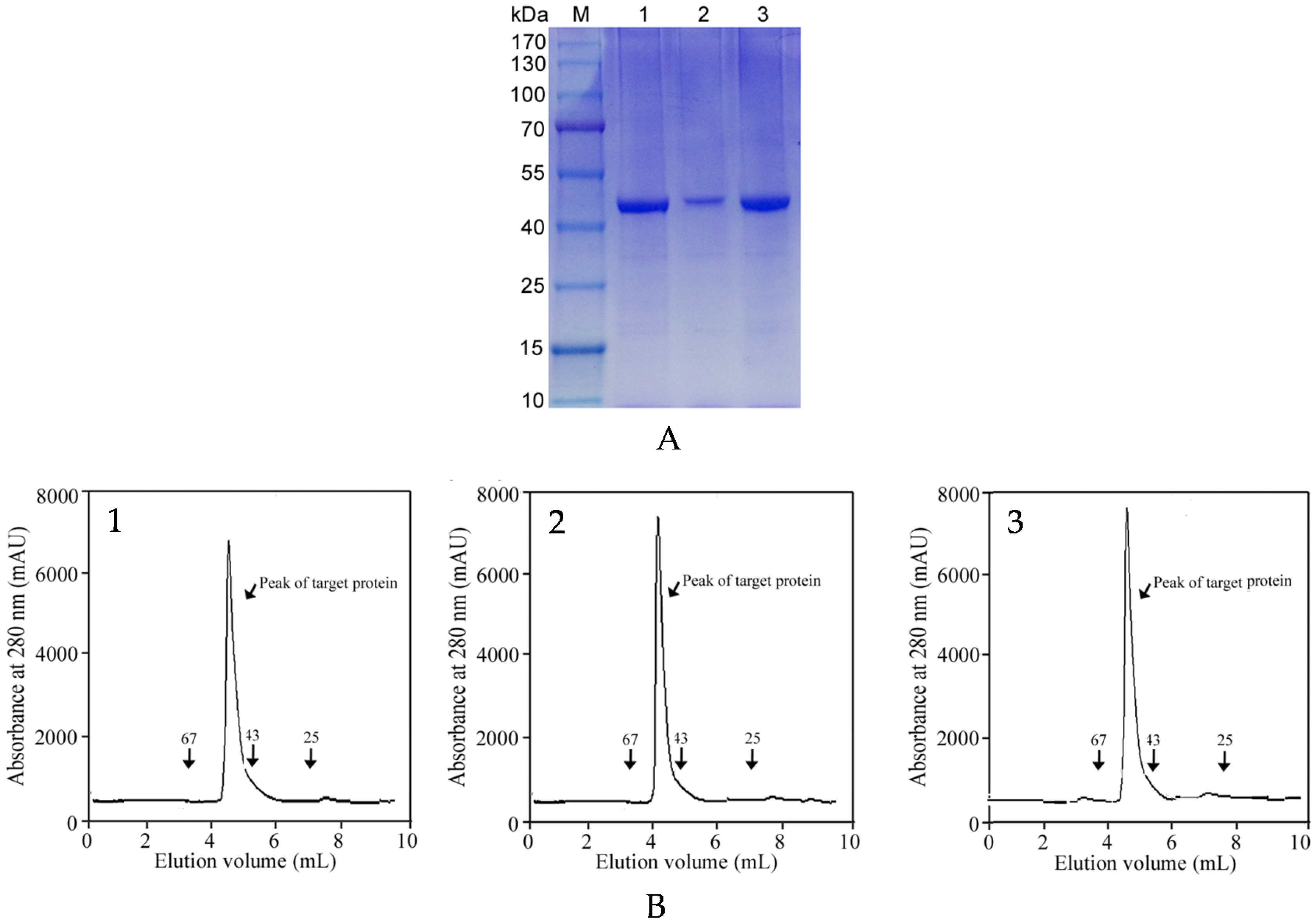
2.3. One-Step Affinity Purification of Commercial Metalloprotease Products
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Affinity Medium
3.3. Adsorption Value Analysis
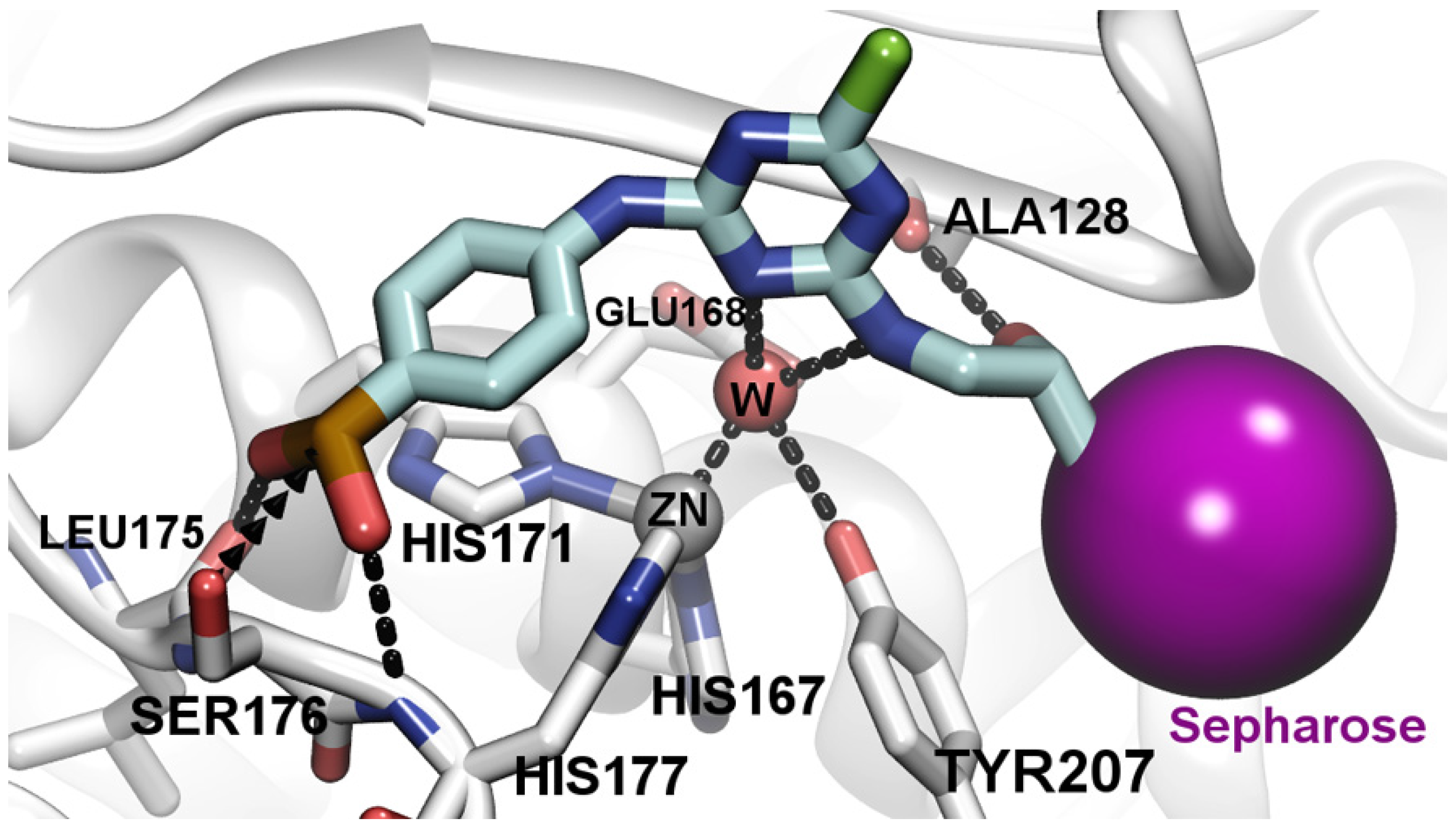
3.4. Molecular Docking Analysis
3.5. Traditional and Affinity Purification of Three Commercial Metalloproteases
3.6. Enzymatic Activity Assay
3.7. Protein Purity Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Q.; Yi, L.; Marek, P.; Iverson, B.L. Commercial proteases: Present and future. FEBS Lett. 2014, 587, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Beg, Q.; Khan, S.; Chauhan, B. An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl. Microbiol. Biotechnol. 2002, 60, 381–395. [Google Scholar] [PubMed]
- Wang, F.; Hao, J.H.; Yang, C.Y.; Sun, M. Cloning, expression, and identification of a novel extracellular cold-adapted alkaline protease gene of the marine bacterium strain YS-80-122. Appl. Biochem. Biotechnol. 2010, 162, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.H.; Sun, M. Purification and characterization of a cold alkaline protease from a psychrophilic Pseudomonas aeruginosa HY1215. Appl. Biochem. Biotechnol. 2015, 175, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Kunamneni, A.; Durvasula, R. Streptokinase—A drug for thrombolytic therapy: A patent review. Recent Adv. Cardiovasc. Drug Discov. 2014, 9, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Craik, C.S.; Page, M.J.; Madison, E.L. Proteases as therapeutics. Biochem. J. 2011, 435, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Yu, X.H.; Wang, C.; Yang, W.; He, W.S.; Zhang, S.J.; Yan, Y.G.; Zhang, J. MMPs and ADAMTSs in intervertebral disc degeneration. Clin. Chim. Acta 2015, 448, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Erban, T. Purification of tropomyosin, paramyosin, actin, tubulin, troponin and kinases for chemiproteomics and its application to different scientific fields. PLoS ONE 2011, 6, e22860. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Suzuki, T.; Hasegawa, M.; Kato, Y.; Sasaki, H.; Inouye, K. Characterization of p-aminobenzamidine-based sorbent and its use for high-performance affinity chromatography of trypsin-like proteases. J. Chromatogr. A 2003, 1009, 133–139. [Google Scholar] [CrossRef]
- Braganza, V.J.; Simmons, W.H. Tryptase from rat skin: Purification and properties. Biochemistry 1991, 30, 4997–5007. [Google Scholar] [CrossRef] [PubMed]
- Burchacka, E.; Witkowska, D. The role of serine proteases in the pathogenesis of bacterial infections. Postep. Hig. Med. Doświadczalnej 2016, 70, 678–694. [Google Scholar] [CrossRef] [PubMed]
- Jean, M.; Raghavan, A.; Charles, M.L.; Robbins, M.S.; Wagner, E.; Rivard, G.É.; Charest-Morin, X.; Marceau, F. The isolated human umbilical vein as a bioassay for kinin-generating proteases: An in vitro model for therapeutic angioedema agents. Life Sci. 2016, 155, 180–188. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Dickstein, K.; Køber, L.V. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N. Engl. J. Med. 2016, 374, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Häse, C.C.; Finkelstein, R.A. Bacterial extracellular zinc-containing metalloproteases. Microbiol. Rev. 1993, 12, 823–837. [Google Scholar]
- Adekoya, O.A.; Sylte, I. The thermolysin family (M4) of enzymes: Therapeutic and biotechnological potential. Chem. Biol. Drug Des. 2009, 73, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Gowda, C.D.; Shivaprasad, H.V.; Kumar, R.V.; Rajesh, R.; Saikumari, Y.K.; Frey, B.M.; Frey, F.J.; Sharath, B.K.; Vishwanath, B.S. Characterization of major zinc containing myonecrotic and procoagulant metalloprotease ‘malabarin’ from non lethal trimeresurus malabaricus snake venom with thrombin like activity: Its neutralization by chelating agents. Curr. Top. Med. Chem. 2011, 11, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.F.; Cui, C.; Zhao, H.F.; Tang, X.L.; Zhao, M.M. Purification and characterization of a new neutral metalloprotease from marine Exiguobacterium sp. SWJS2. Biotechnol. Appl. Biochem. 2016, 63, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Banik, S.P.; Khowala, S. Purification and characterisation of κ-casein specific milk-clotting metalloprotease from Termitomyces clypeatus MTCC 5091. Food Chem. 2015, 173, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Yang, J.; Chen, X.L.; Su, H.N.; Zhang, X.Y.; Huang, F.; Zhou, B.C.; Xie, B.B. Optimization of fermentation conditions for the production of the M23 protease Pseudoalterin by deep-sea Pseudoalteromonas sp. CF6-2 with artery powder as an inducer. Molecules 2014, 19, 4779–4790. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Ran, L.Y.; Liu, C.; Chen, X.L.; Zhang, X.Y.; Qin, Q.L.; Zhou, B.C.; Zhang, Y.Z. Culture condition optimization and pilot scale production of the M12 metalloprotease myroilysin produced by the deep-sea bacterium Myroides profundi D25. Molecules 2015, 20, 11891–11901. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, L.N.; Yang, J.; Liu, J.Z.; Lin, S.X.; Hao, J.H.; Sun, M. Affinity purification of metalloprotease from marine bacterium using immobilized metal affinity chromatography. J. Sep. Sci. 2016, 39, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Chessa, J.P.; Petrescu, I.; Bentahir, M.; Van Beeumen, J.; Gerday, C. Purification, physico-chemical characterization and sequence of a heat labile alkaline metalloprotease isolated from a psychrophilic Pseudomonas species. Biochim. Biophys. Acta 2000, 1479, 265–274. [Google Scholar] [CrossRef]
- Martínez, C.A.; Seidel-Morgenstern, A. Purification of single-chain antibody fragments exploiting pH-gradients in simulated moving bed chromatography. J. Chromatogr. A 2016, 1434, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, R.; Li, S.Y.; Chen, X.L.; Yang, K.D.; Chen, G.L.; Ma, X.X. Evaluation and optimization of the metal-binding properties of a complex ligand for immobilized metal affinity chromatography. J. Sep. Sci. 2016, 39, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Wong, J.H.; Ng, T.B. Immobilized metal ion affinity chromatography: A review on its applications. Appl. Microbiol. Biotechnol. 2012, 96, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Labrou, N.E. Design and selection of ligands for affinity chromatography. J. Chromatogr. B 2003, 790, 67–78. [Google Scholar] [CrossRef]
- Ye, L.; Xu, A.Z.; Cheng, C.; Zhang, L.; Huo, C.X.; Huang, F.Y.; Xu, H.; Li, R.Y. Design and synthesis of affinity ligands and relation of their structure with adsorption of proteins. J. Sep. Sci. 2011, 34, 3145–3150. [Google Scholar] [CrossRef] [PubMed]
- Havlicek, V.; Lemr, K.; Schug, K.A. Current trends in microbial diagnostics based on mass spectrometry. Anal. Chem. 2013, 85, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Sun, M.; Li, T.; Wang, Q.H.; Hao, J.H.; Han, Y.; Hu, X.J.; Zhou, M.; Lin, S.X. Structure analysis of a new psychrophilic marine protease. PLoS ONE 2011, 6, e26939. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.F.; Zheng, Y.; Wang, W.; Sheng, J.; Hao, J.H.; Sun, M. Virtual screening of novel reversible inhibitors for marine alkaline protease MP. J. Mol. Graph. Model. 2013, 46, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Chen, Y.; Liu, Z. Boronate affinity materials for separation and molecular recognition: Structure, properties and applications. Chem. Soc. Rev. 2015, 44, 8097–8123. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, J.; Li, X.; Liu, Z. Boronate affinity fluorescent nanoparticles for förster resonance energy transfer inhibition assay of cis-diol biomolecules. Anal. Chem. 2016, 88, 5088–5096. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Shi, W.; Zhu, B.; Gu, X.; Wang, Y.; Yan, C. Polyethyleneimine-grafted boronate affinity materials for selective enrichment of cis-diol-containing compounds. Talanta 2015, 140, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Toprak, A.; Görgün, C.; Kuru, C.İ.; Türkcan, C.; Uygun, M.; Akgöl, S. Boronate affinity nanoparticles for RNA isolation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 50, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.P.; Qi, C.B.; Chu, J.M.; Yuan, B.F.; Feng, Y.Q. Profiling of cis-diol-containing nucleosides and ribosylated metabolites by boronate-affinity organic-silica hybrid monolithic capillary liquid chromatography/mass spectrometry. Sci. Rep. 2015, 5, 7785. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, E.; Reyes, Y.R.; Guzman, O.M.; Rosado, A.; Cruz, V.R.; Borges, A.; Martinez, E.; Vibha Bansal, V. Para-aminobenzamidine linked regenerated cellulose membranes for plasminogen activator purification: Effect of spacer arm length and ligand density. J. Chromatogr. B 2013, 930, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Dong, D.X.; Wang, T.; Li, R.X. Affinity purification of serine proteinase from Deinagkistrodon acutus venom. J. Chromatogr. B 2007, 859, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Van Ness, J.; Kalbfleisch, S.; Petrie, C.R.; Reed, M.W.; Tabone, J.C.; Vermeulen, N.M. A versatile solid support system for oligodeoxynucleotide probe-based hybridization assays. Nucleic Acids. Res. 1991, 19, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Bhushan, R. Amino acids as chiral auxiliaries in cyanuric chloride-based chiral derivatizing agents for enantioseparation by liquid chromatography. Biomed. Chromatogr. 2014, 28, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.E.; Li, K.L.; Lin, P.Y.; Lin, J.L. Infrared spectroscopic study of the adsorption forms of cyanuric acid and cyanuric chloride on TiO2. Langmuir 2016, 32, 5306–5313. [Google Scholar] [CrossRef] [PubMed]
- Mountford, S.J.; Daly, R.; Robinson, A.J.; Hearn, M.T. Design, synthesis and evaluation of pyridine-based chromatographic adsorbents for antibody purification. J. Chromatogr. A 2014, 1355, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Smoum, R.; Rubinstein, A.; Dembitsky, V.M.; Srebnik, M. Boron containing compounds as protease inhibitors. Chem. Rev. 2012, 112, 4156–4220. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Pan, S.H.; Chen, G.G.; Huang, S.; Zhang, Z.F.; Li, Y.; Liang, Z.Q. Biochemical characteristics of a fibrinolytic enzyme purified from a marine bacterium, Bacillus subtilis HQS-3. Int. J. Biol. Macromol. 2013, 62, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Q.; Zhong, L.F.; Meng, Z.Z.; You, Z.J.; Li, J.Z.; Luo, X.C. The structure and enzyme characteristics of a recombinant leucine aminopeptidase rlap1 from Aspergillus sojae and its application in debittering. Appl. Biochem. Biotechnol. 2015, 177, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ruckenstein, E. A new matrix for membrane affinity chromatography and its application to the purification of concanavalin A. J. Membr. Sci. 2001, 182, 227–234. [Google Scholar] [CrossRef]
- Dong, D.X.; Liu, H.R.; Xiao, Q.S.; Li, R.X. Affinity purification of egg yolk immunoglobulins (IgY) with a stable synthetic ligand. J. Chromatogr. B 2008, 870, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yang, H.L.; Xiao, X.L.; Zhang, L.; Zhang, Y.R.; Tong, Y.J.; Chen, Y.; Wang, W. Affinity purification of urinary trypsin inhibitor from human urine. J. Sep. Sci. 2012, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Santos-Martins, D.; Forli, S.; Ramos, M.J.; Olson, A.J. AutoDock4(Zn): An improved AutoDock force field for small-molecule docking to zinc metalloproteins. J. Chem. Inf. Model. 2014, 54, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
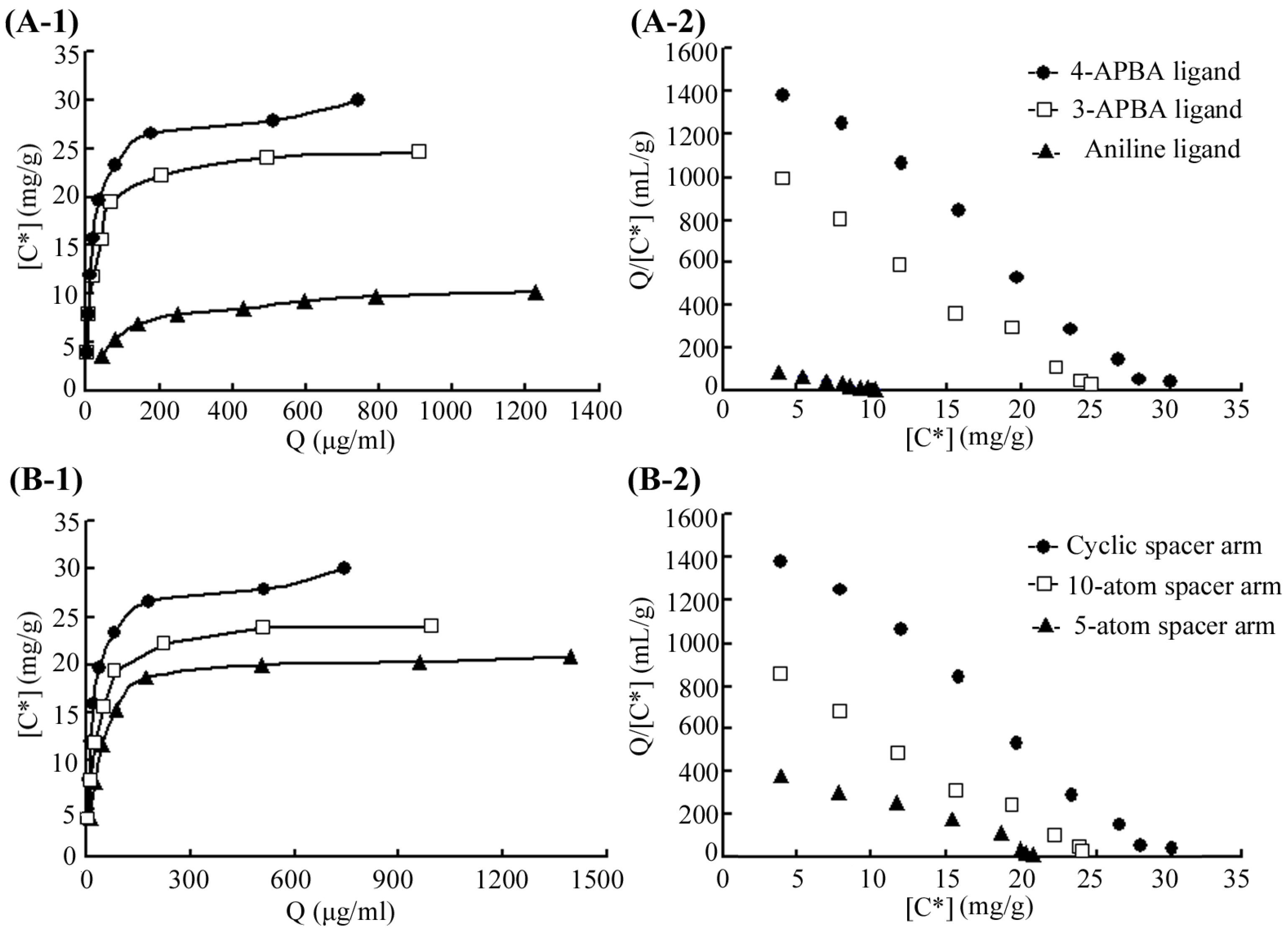
| Ligands | Spacer Arms | Ligand Density (μmol/mL) | Kd (μg/mL) | Qmax (mg/g) |
|---|---|---|---|---|
| Aniline | Cyanuric chloride | 20.9 | 67.2 | 10.6 |
| 3-APBA a | Cyanuric chloride | 20.9 | 21.5 | 24.9 |
| 4-APBA | Cyanuric chloride | 20.9 | 14.9 | 29.6 |
| 4-APBA | 10-atom spacer | 41.8 | 24.4 | 24.6 |
| 4-APBA | 5-atom spacer | 27.8 | 46.3 | 22.3 |
| Enzymes | Purification Method | Activity Recovery (%) | Protein Purity (%) | Specific Activity (U/mg) | Time Requirement |
|---|---|---|---|---|---|
| MP | Affinity protocol a | 64.1 | 98.8 | 95.6 | ~1 h |
| Traditional protocol b | 8.9 | 97.6 | 96.2 | >48 h | |
| DENIE-B LPS-P | Affinity protocol a | 45.2 | 95.9 | 64.6 | ~1 h |
| Traditional protocol c | 10.7 | 91.4 | 62.3 | >48 h | |
| ViscozymeL | Affinity protocol a | 37.8 | 97.1 | 51.4 | ~1 h |
| Traditional protocol d | 3.4 | 58.3 | 27.8 | >96 h |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, L.; Xu, X.; Lin, S.; Wang, Y.; Hao, J.; Sun, M. Structure-Based Design and Synthesis of a New Phenylboronic-Modified Affinity Medium for Metalloprotease Purification. Mar. Drugs 2017, 15, 5. https://doi.org/10.3390/md15010005
Li S, Wang L, Xu X, Lin S, Wang Y, Hao J, Sun M. Structure-Based Design and Synthesis of a New Phenylboronic-Modified Affinity Medium for Metalloprotease Purification. Marine Drugs. 2017; 15(1):5. https://doi.org/10.3390/md15010005
Chicago/Turabian StyleLi, Shangyong, Linna Wang, Ximing Xu, Shengxiang Lin, Yuejun Wang, Jianhua Hao, and Mi Sun. 2017. "Structure-Based Design and Synthesis of a New Phenylboronic-Modified Affinity Medium for Metalloprotease Purification" Marine Drugs 15, no. 1: 5. https://doi.org/10.3390/md15010005
APA StyleLi, S., Wang, L., Xu, X., Lin, S., Wang, Y., Hao, J., & Sun, M. (2017). Structure-Based Design and Synthesis of a New Phenylboronic-Modified Affinity Medium for Metalloprotease Purification. Marine Drugs, 15(1), 5. https://doi.org/10.3390/md15010005






