Indolediketopiperazine Alkaloids from Eurotium cristatum EN-220, an Endophytic Fungus Isolated from the Marine Alga Sargassum thunbergii
Abstract
:1. Introduction
2. Results and Discussion
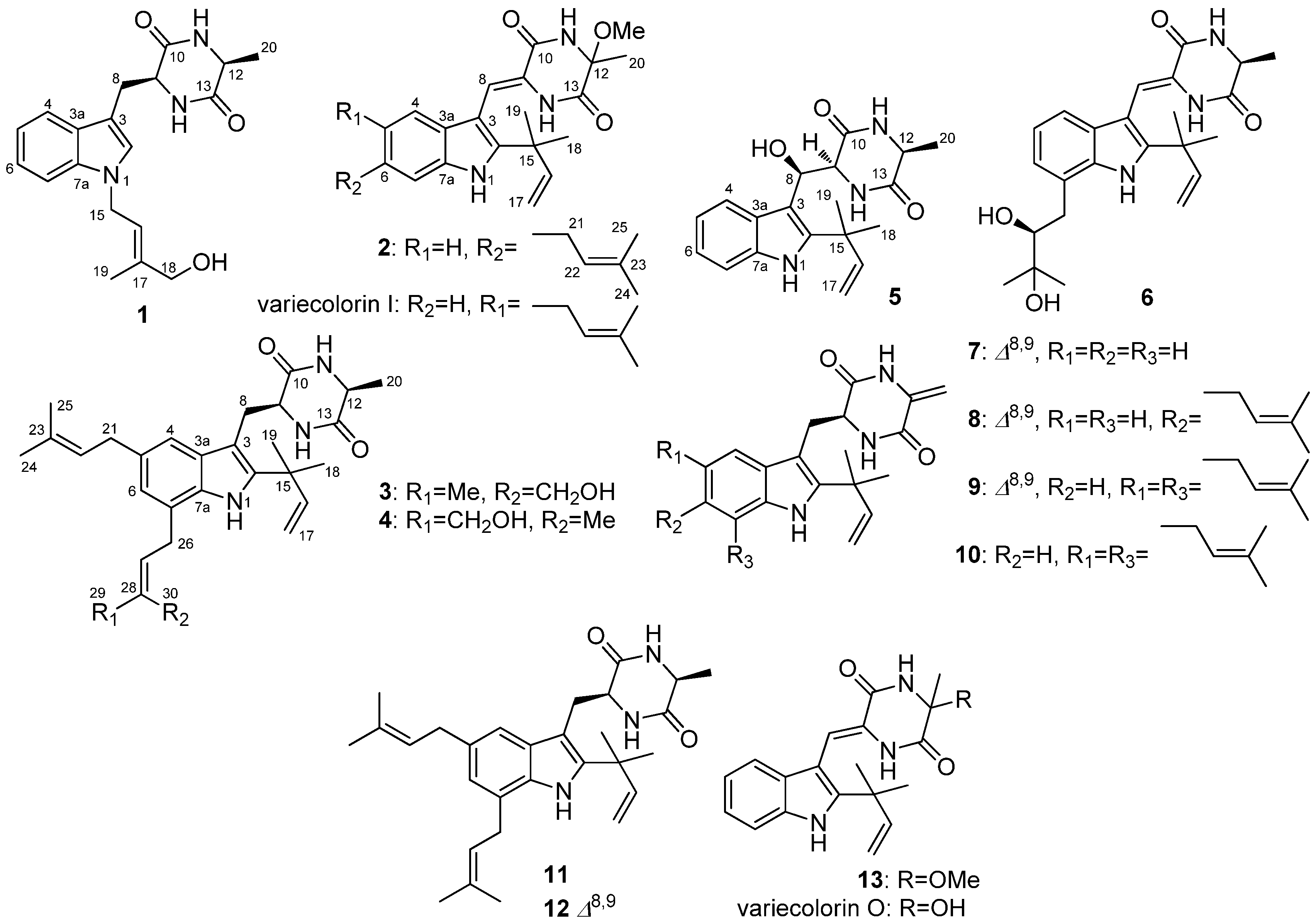
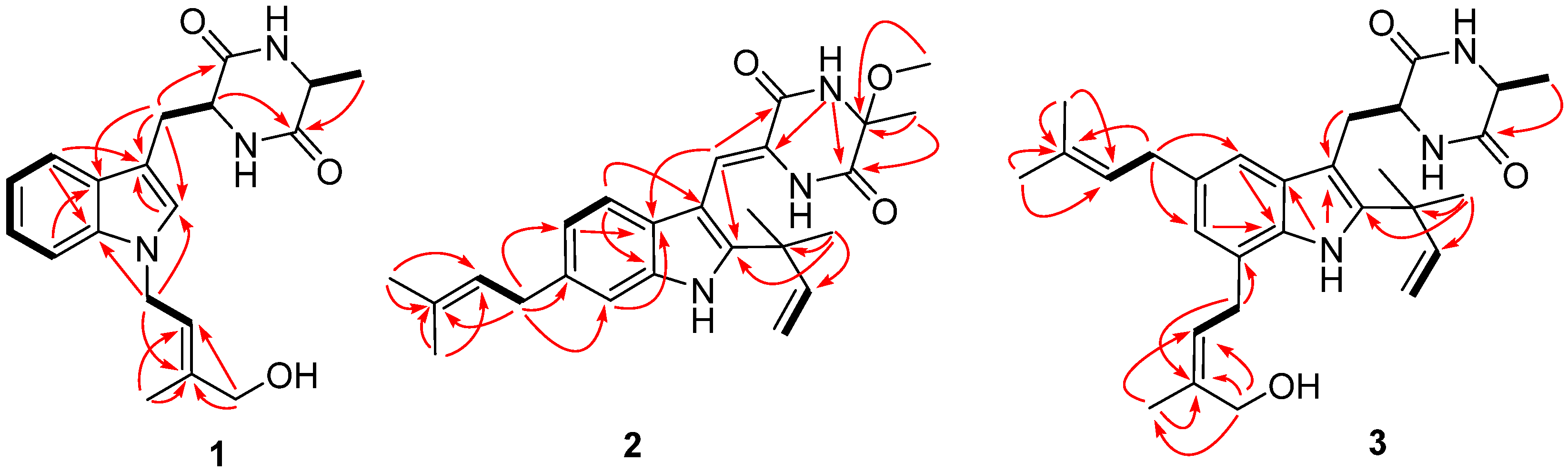
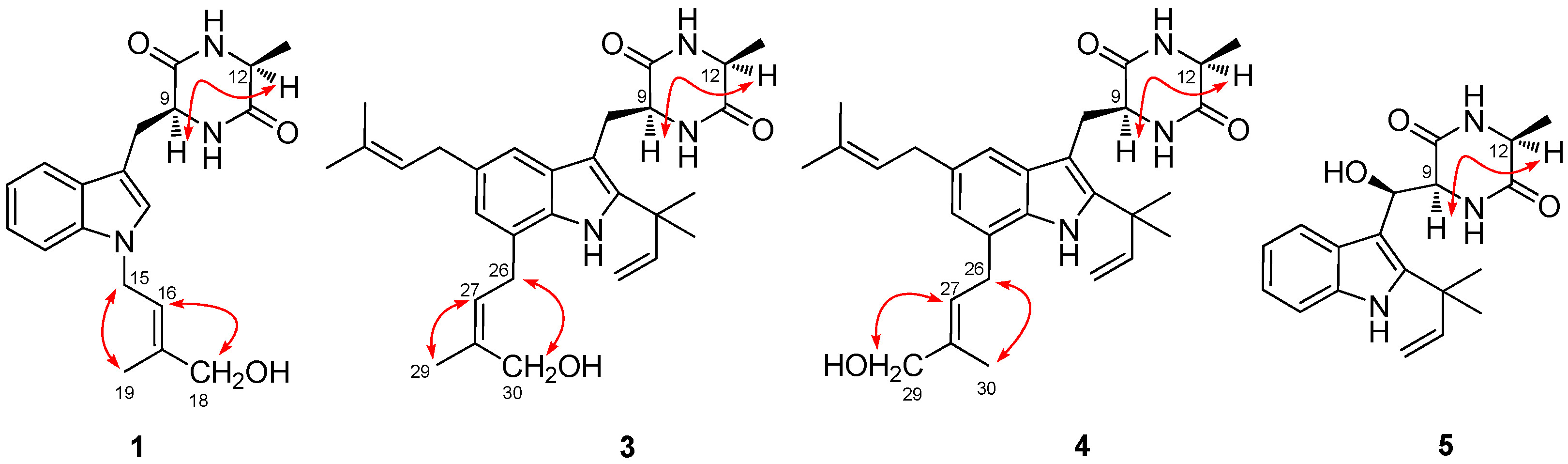
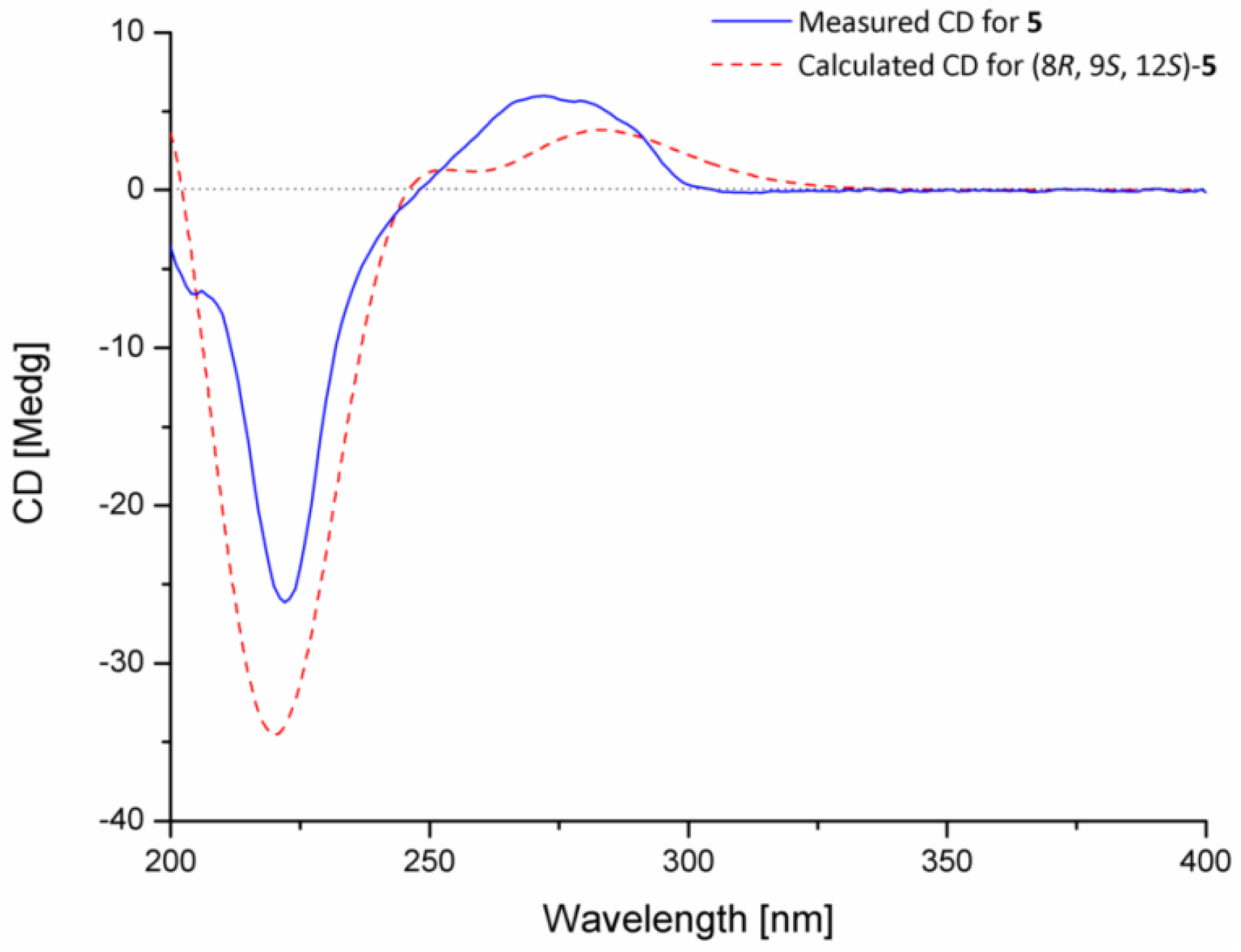
2.1. Structural Elucidation of Indolediketopiperazines
2.2. Bioactivities of Indolediketopiperazines
3. Experimental Section
3.1. General Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. ECD Calculation of Compound 5
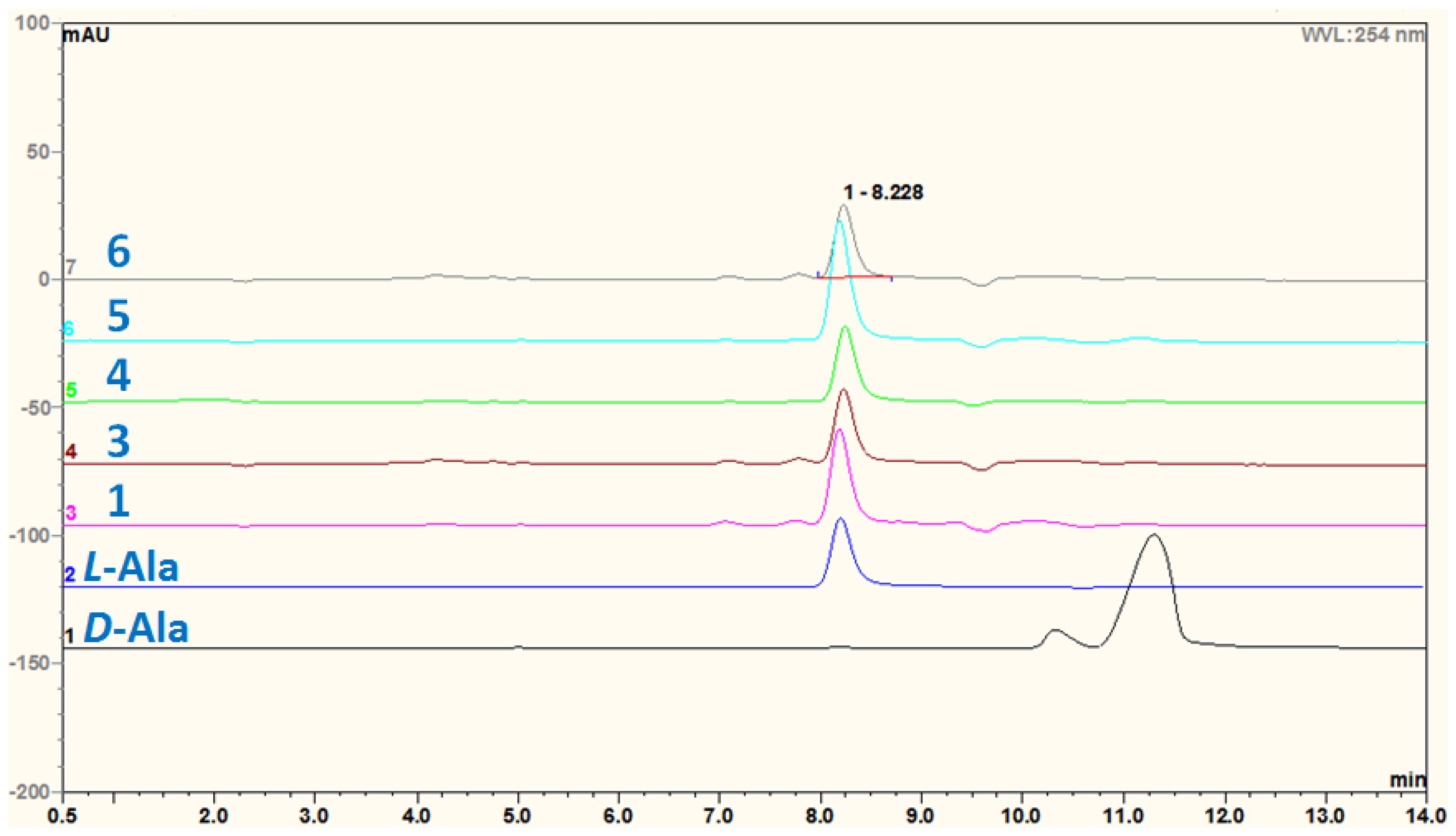
3.5. Acidic Hydrolysis of Compounds 1 and 3–6
3.6. Verification of Compound 2 Being Not an Artifact
3.7. Bioassay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ji, N.Y.; Wang, B.G. Mycochemistry of marine algicolous fungi. Fungal Divers. 2016, 80, 301–342. [Google Scholar] [CrossRef]
- Abdel-Gawad, K.M.; Hifney, A.F.; Issa, A.A.; Gomaa, M. Spatio-temporal, environmental factors, and host identity shape culturable-epibiotic fungi of seaweeds in the Red Sea, Egypt. Hydrobiologia 2014, 740, 37–49. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.M.; Li, C.S.; Shang, Z.; Wang, B.G. Cristatumins A–D, new indole alkaloids from the marine-derived endophytic fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 2012, 22, 4650–4653. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, L.; Gnavi, G.; Varese, G.C.; Picco, A.M. Mycobiota associated with the rhodophyte alien species Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon in the Mediterranean Sea. Mar. Ecol.-Evolut. Perspect. 2015, 36, 959–968. [Google Scholar] [CrossRef]
- Felício, R.; Pavão, G.B.; Oliveira, A.L.L.; Erbert, C.; Conti, R.; Pupo, M.T.; Furtado, N.A.J.C.; Ferreira, E.G.; Costa-Lotufo, L.V.; Young, M.C.M.; et al. Antibacterial, antifungal and cytotoxic activities exhibited by endophytic fungi from the Brazilian marine red alga Bostrychia tenella (Ceramiales). Rev. Bras. Farmacogn. 2015, 25, 641–650. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.L.; Wang, Y.; Fu, P.; Liu, P.P.; Wang, C.; Zhu, W.M. A cytotoxic pyrrolidinoindoline diketopiperazine dimer from the algal fungus Eurotium herbariorum HT-2. Chin. Chem. Lett. 2013, 24, 1049–1052. [Google Scholar] [CrossRef]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Wang, B.G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2008, 91, 1888–1893. [Google Scholar] [CrossRef]
- Yan, H.J.; Li, X.M.; Li, C.S.; Wang, B.G. Alkaloid and anthraquinone derivatives produced by the marine-derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2012, 95, 163–168. [Google Scholar] [CrossRef]
- Meng, L.H.; Du, F.Y.; Li, X.M.; Pedpradab, P.; Xu, G.M.; Wang, B.G. Rubrumazines A–C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 2015, 78, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.M.; Liang, X.A.; Kong, Y.; Jia, B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; He, H.P.; Shen, Y.M.; Zhang, K.Q. Nematicidal epipolysulfanyldioxopiperazines from Gliocladium roseum. J. Nat. Prod. 2005, 68, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Lu, Z.Y.; Tao, H.W.; Zhu, T.J.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Isoechinulin-type alkaloids, variecolorins A–L, from halotolerant Aspergillus variecolor. J. Nat. Prod. 2007, 70, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.N.; Zhu, T.J.; Cai, S.X.; Gu, Q.Q.; Li, D.H. Three new indole-containing diketopiperazine alkaloids from a deep-ocean sediment derived fungus Penicillium griseofulvum. Helv. Chim. Acta 2010, 93, 1758–1763. [Google Scholar] [CrossRef]
- Fujimoto, H.; Fujimaki, T.; Okuyama, E.; Yamazaki, M. Immunomodulatory constituents from an ascomycete, Microascustardifaciens. Chem. Pharm. Bull. 1999, 47, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Jiang, L.H.; Guo, L.D.; Chen, X.L.; Zhang, H.; Che, Y.S. Pestalazines and pestalamides, bioactive metabolites from the plant pathogenic fungus Pestalotiopsis theae. J. Nat. Prod. 2008, 71, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Q.; Zhu, T.J.; Li, D.H.; Gu, Q.Q.; Liu, W.Z. Prenylated indole diketopiperazine alkaloids from a mangroverhizosphere soil-derived fungus Aspergillus effuses H1-1. Arch. Pharm. Res. 2013, 36, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Zhang, P.; Li, X.M.; Wang, B.G. Penicibrocazines A–E, five new sulfide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Mar. Drugs 2015, 13, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mándi, A.; Li, X.M.; Meng, L.H.; Kurtán, T.; Wang, B.G. Peniciadametizine A, a dithiodiketopiperazine with a unique spiro[furan-2,7′-pyrazino[1,2-b][1,2]oxazine] skeleton, and a related analogue, peniciadametizine B, from the marine sponge-derived fungus Penicillium adametzioides. Mar. Drugs 2015, 13, 3640–3652. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.M.; Liu, Y.; Zhang, P.; Wang, J.N.; Wang, B.G. Chermesins A–D: Meroterpenoids with a drimane-type spirosesquiterpene skeleton from the marine algal-derived endophytic fungus Penicillium chermesinum EN-480. J. Nat. Prod. 2016, 79, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Li, X.M.; Liu, Y.; Wang, B.G. Penicibilaenes A and B, sesquiterpenes with a tricyclo[6.3.1.0(1,5)]dodecane skeleton from the marine isolate of Penicillium bilaiae MA-267. Org. Lett. 2014, 16, 6052–6055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Mándi, A.; Li, X.M.; Du, F.Y.; Wang, J.N.; Li, X.; Kurtán, T.; Wang, B.G. Varioxepine A, a 3H-oxepine-containing alkaloid with a new oxa-cage from the marine algal-derived endophytic fungus Paecilomyces variotii. Org. Lett. 2014, 16, 4834–4837. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.W.; Li, Y.; Zhang, X.N.; Li, Q.; Liu, X.; Huang, Y.; Tang, T.; Zheng, S.J.; Wang, W.M.; Tang, J.T. A new prenylated indole diketopiperazine alkaloid from Eurotium cristatum. Molecules 2014, 19, 17839–17847. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L. Secondary Metabolites and Their Bioactivities of a Hibiscus tiliaceus-Derived Endophytic Fungus Eurotium rubrum and a Mangrove Plant Rhizophora stylosa Griff. Ph.D. Thesis, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China, June 2008. [Google Scholar]
- Marchelli, R.; Dossena, A.; Pochini, A.; Dradi, E. The structures of five new didehydropeptides related to neoechinulin, isolated from Aspergillus amstelodami. J. Chem. Soc. Perkin Trans. 1 1977, 7, 713–717. [Google Scholar] [CrossRef]
- Matthew, S.; Paul, V.J.; Luesch, H. Tiglicamides A–C, cyclodepsipeptides from the marine cyanobacterium Lyngbya confervoides. Phytochemistry 2009, 70, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Si, L.L.; Liu, D.; Proksch, P.; Zhang, L.H.; Zhou, D.M.; Lin, W.H. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, T.; Matsuishi, N.; Hosoe, T.; Toyazaki, N.; Udagawa, S.; Imai, T.; Adachi, M.; Kawai, K. Two new dioxopiperazine derivatives, arestrictins A and B, isolated from Aspergillus restrictus and Aspergillus penicilloides. Chem. Pharm. Bull. 2006, 54, 1639–1641. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013.
- Wang, W.L. Studies on Halotolerant Fungal Strains: Isolation, Culture and Secondary Metabolites. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2008. [Google Scholar]
- Wang, Z.Q.; Zhou, H.; Han, J.; He, Y.Q.; Lu, L.P. Evaluate insecticidal activity using Artemia salina L. Agrochemicals 2011, 50, 261–263. [Google Scholar]
- Yao, C.C. Development and Application of Artemia nauplii Screening Method for Insecticidal Activities of Bamboo Extracts. Master’s Thesis, Anhui Agricultural University, Hefei, China, June 2009. [Google Scholar]
- Choi, H.J.; Engene, N.; Smith, J.E.; Preskitt, L.B.; Gerwick, W.H. Crossbyanols A–D, toxic brominated polyphenyl ethers from the Hawaiian bloom-forming Cyanobacterium Leptolyngbya crossbyana. J. Nat. Prod. 2010, 73, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kivcak, B.; Mert, T. Preliminary evaluation of cytotoxic properties of Laurus nobilis leaf extracts. Fitoterapia 2002, 73, 242–243. [Google Scholar] [CrossRef]
- Lu, Q.; Shen, Q.Y.; Sun, L.; Wang, J.Y.; Song, G.H. Synthesis and nematicidal activities of 8-azabicyclo[3.2.1]-octane-3-isoxazole oxime derivatives. Chin. J. Org. Chem. 2016, 36, 760–767. [Google Scholar] [CrossRef]
| Compd. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| brine shrimp lethality | n.a. | 19.4 | 138.1 | 140.6 | n.a. | n.a. | 105.2 | 70.1 | 19.8 | 27.1 | n.a. | n.a. | n.a. |
| nematicidal activity | n.a. | 110.3 | n.a. | n.a. | n.a. | n.a. | >200 | >200 | 106.7 | 126.4 | n.a. | n.a. | n.a. |
| Compd. | 2 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Ascorbic Acid |
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | 20.6 | 28.5 | 10.9 | 12.1 | 10.1 | 13.3 | 13.8 | 6.4 | 18.7 | 2.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, F.-Y.; Li, X.; Li, X.-M.; Zhu, L.-W.; Wang, B.-G. Indolediketopiperazine Alkaloids from Eurotium cristatum EN-220, an Endophytic Fungus Isolated from the Marine Alga Sargassum thunbergii. Mar. Drugs 2017, 15, 24. https://doi.org/10.3390/md15020024
Du F-Y, Li X, Li X-M, Zhu L-W, Wang B-G. Indolediketopiperazine Alkaloids from Eurotium cristatum EN-220, an Endophytic Fungus Isolated from the Marine Alga Sargassum thunbergii. Marine Drugs. 2017; 15(2):24. https://doi.org/10.3390/md15020024
Chicago/Turabian StyleDu, Feng-Yu, Xin Li, Xiao-Ming Li, Li-Wei Zhu, and Bin-Gui Wang. 2017. "Indolediketopiperazine Alkaloids from Eurotium cristatum EN-220, an Endophytic Fungus Isolated from the Marine Alga Sargassum thunbergii" Marine Drugs 15, no. 2: 24. https://doi.org/10.3390/md15020024






