Discovery of Novel Bromophenol Hybrids as Potential Anticancer Agents through the Ros-Mediated Apoptotic Pathway: Design, Synthesis and Biological Evaluation
Abstract
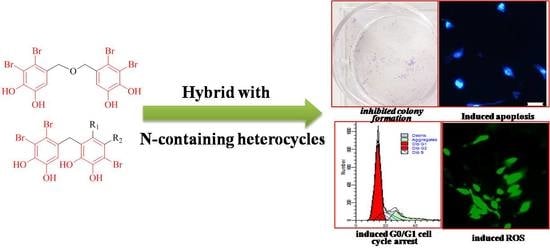
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activity
2.2.1. Cytotoxicity
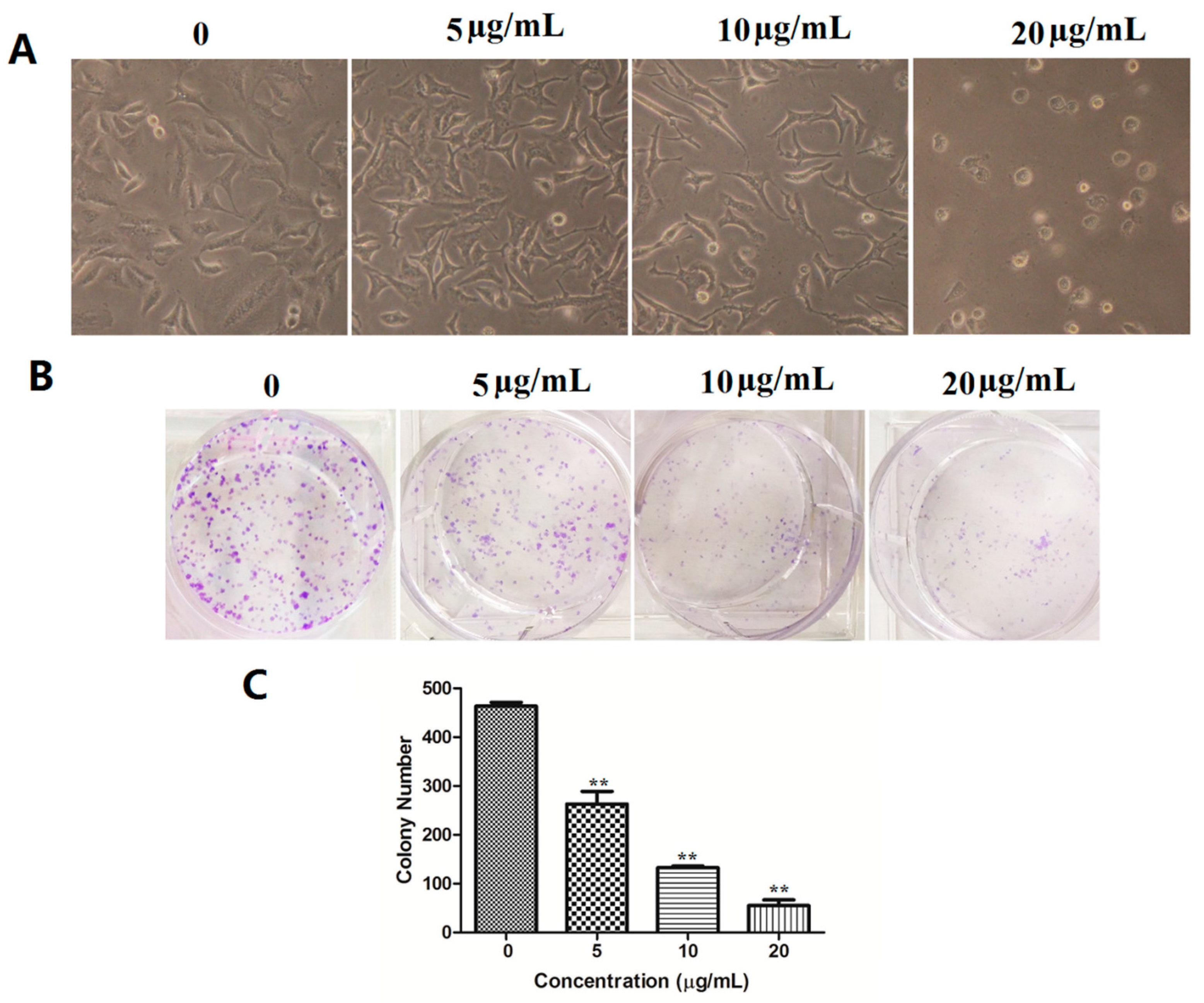
2.2.2. Compound 17a Induce Morphological Changes in A549 Cells
2.2.3. Compound 17a Inhibits Colony Formation Ability of A549 Cells
2.2.4. Compound 17a Induce Apoptosis in A549 Cells
2.2.5. Compound 17a Causes DNA Fragmentations and Morphological Changes
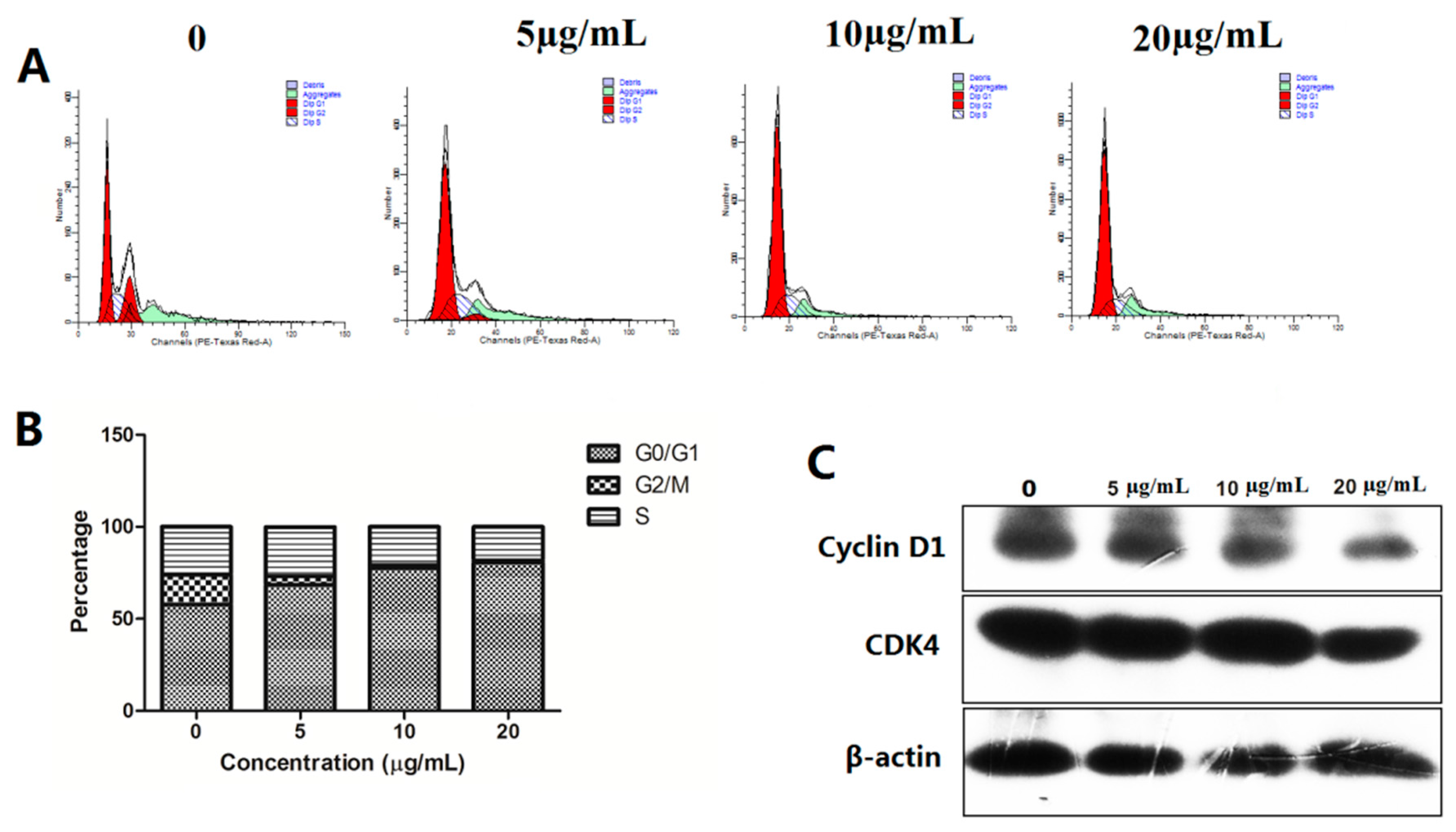
2.2.6. Compound 17a Induce G0/G1 Cell Cycle Arrest in A549 Cells
2.2.7. Compound 17a Triggers ROS Generation
2.2.8. Effect of Compound 17a on the Expression of Apoptosis-Related Proteins
3. Materials and Methods
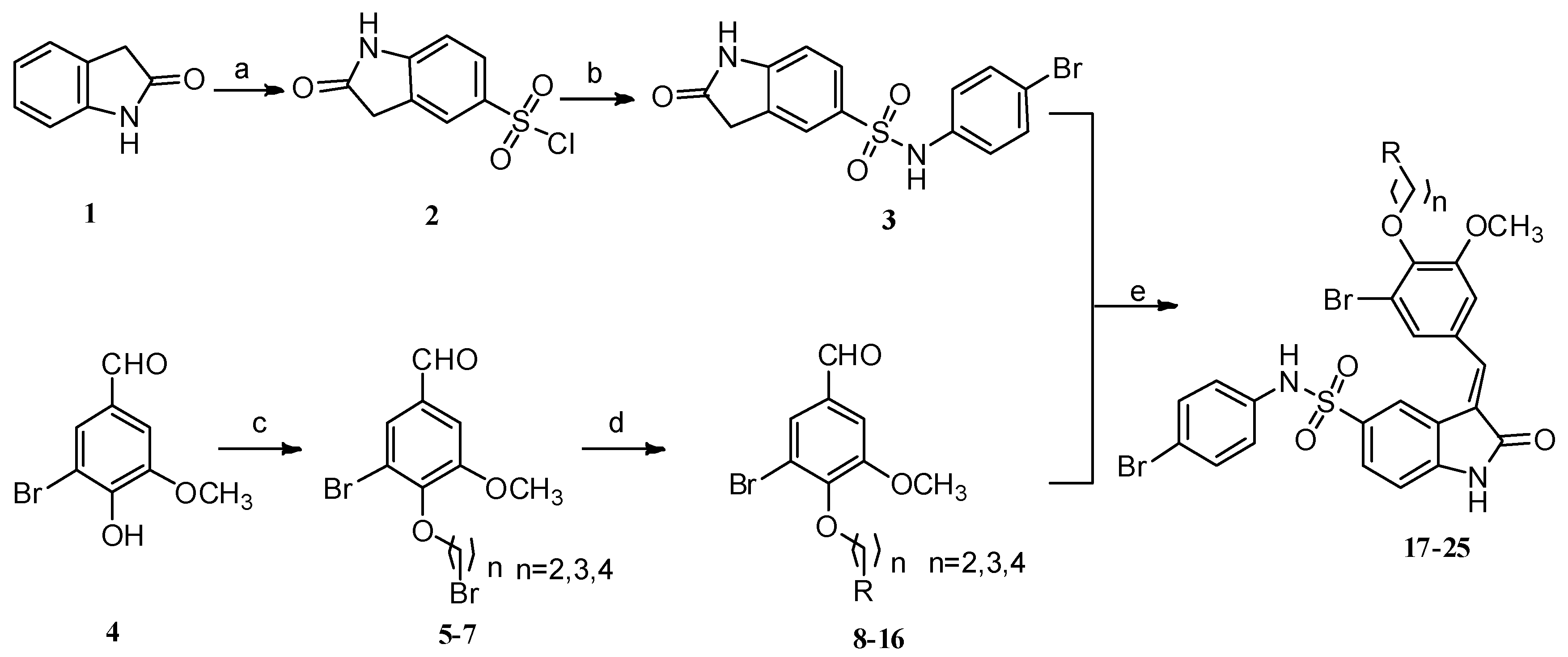
3.1. Chemistry
3.1.1. General Procedures for the Preparation of Compounds 5–7
3.1.2. General Procedure for the Preparation of Compounds 8–16
3.1.3. General Procedures for the Preparation of Compounds 17–25
3.2. Biological Activity
3.2.1. Cell Culture
3.2.2. MTT Assay
3.2.3. Colony Forming Assay
3.2.4. Cell Cycle Analysis
3.2.5. Cell Apoptosis Analysis
3.2.6. Apoptosis Assessment by Hoechst 33258 Staining
3.2.7. Reactive Oxygen Species (ROS) Levels Assay
3.2.8. Western Blot Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kao, S.J.; Lee, W.J.; Chang, J.H.; Chow, J.M.; Chung, C.L.; Hung, W.Y.; Chien, M.H. Suppression of reactive oxygen species-mediated ERK and JNK activation sensitizes dihydromyricetin-induced mitochondrial apoptosis in human non-small cell lung cancer. Environ. Toxicol. 2017, 32, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.H.; Siraj, S.; Arshad, A.; Waheed, U.; Aldakheel, F.; Alduraywish, S.; Arshad, M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1789–1809. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Wang, L.L.; Wang, X.B.; Xi, T.; Liao, J.M.; Wang, Z.X.; Jiang, F. Design, combinatorial synthesis and biological evaluations of novel 3-amino-1′-((1-aryl-1H-1,2,3-triazol-5-yl)methyl)-2′-oxospiro[benzo [a] pyrano[2,3-c]phenazine-1,3′-indoline]-2-carbonitrile antitumor hybrid molecules. Eur. J. Med. Chem. 2017, 135, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Manikandamathavan, V.M.; Thangaraj, M.; Weyhermuller, T.; Parameswari, R.P.; Punitha, V.; Murthy, N.N.; Nair, B.U. Novel mononuclear Cu(II) terpyridine complexes: Impact of fused ring thiophene and thiazole head groups towards DNA/BSA interaction, cleavage and antiproliferative activity on HepG2 and triple negative CAL-51 cell line. Eur. J. Med. Chem. 2017, 135, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.X.; Zou, B.Y.; Shi, L.; Du, L.Y.; Zhu, Y.Y.; Jiang, Y.M.; Ma, X.D.; Kang, X.H.; Wang, C.Y.; Zhen, Y.H.; et al. 7-O-geranylquercetin-induced autophagy contributes to apoptosis via ROS generation in human non-small cell lung cancer cells. Life Sci. 2017, 180, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Wang, S.Y.; Jiang, B.; Wu, N.; Li, X.Q.; Wang, B.C.; Luo, J.; Yang, M.; Jin, S.H.; Shi, D.Y. Design, Synthesis and Biological Evaluation of Novel Bromophenol Derivatives Incorporating Indolin-2-One Moiety as Potential Anticancer Agents. Mar. Drugs 2015, 13, 806–823. [Google Scholar] [CrossRef] [PubMed]
- Sherer, C.; Snape, T.J. Heterocyclic scaffolds as promising anticancer agents against tumours of the central nervous system: Exploring the scope of indole and carbazole derivatives. Eur. J. Med. Chem. 2015, 97, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wang, L.J.; Jiang, B.; Wu, N.; Li, X.Q.; Luo, J.; Wang, B.C.; Zhang, R.S.; Xu, Q.; Shi, D.Y. Synthesis and biological evaluation of novel fluorinated anticancer agents incorporating the indolin-2-one moiety. RSC Adv. 2015, 5, 91795–91801. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, M.; Holzer, W. Synthesis and evaluation of indole, pyrazole, chromone and pyrimidine based conjugates for tumor growth inhibitory activities—Development of highly efficacious cytotoxic agents. Eur. J. Med. Chem. 2010, 45, 4968–4982. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Zhao, Y.F.; Zhang, G.G.; Lv, Y.X.; Zhang, N.; Gong, P. Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent. Eur. J. Med. Chem. 2011, 46, 3509–3518. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.S.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, S.H.; Wang, C.T.; Li, Z.H.; Ma, Y.; Liu, G. Antagonizing NOD2 Signaling with Conjugates of Paclitaxel and Muramyl Dipeptide Derivatives Sensitizes Paclitaxel Therapy and Significantly Prevents Tumor Metastasis. J. Med. Chem. 2017, 60, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Alza, N.P.; Richmond, V.; Baier, C.J.; Freire, E.; Baggio, R.; Murray, A.P. Synthesis and cholinesterase inhibition of cativic acid derivatives. Bioorg. Med. Chem. 2014, 22, 3838–3849. [Google Scholar] [CrossRef] [PubMed]
- Taddei, M.; Ferrini, S.; Giannotti, L.; Corsi, M.; Manetti, F.; Giannini, G.; Vesci, L.; Milazzo, F.M.; Alloatti, D.; Guglielmi, M.B.; et al. Synthesis and Evaluation of New Hsp90 Inhibitors Based on a 1,4,5-Trisubstituted 1,2,3-Triazole Scaffold. J. Med. Chem. 2014, 57, 2258–2274. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Ma, L.; Huang, W.X.; Yu, X.; Zhang, Y.H.; Ji, H.; Tian, J.D. Synthesis and biological evaluation of 3-[4-(amino/methylsulfonyl) phenyl]methylene-indolin-2-one derivatives as novel COX-1/2 and 5-LOX inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 7349–7353. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.H.; Shen, J.J.; Zhan, Y.C.; Yi, H.; Xue, S.T.; Wang, Z.; Ji, X.Y.; Li, Z.R. Design, synthesis and in vitro and in vivo antitumour activity of 3-benzylideneindolin-2-one derivatives, a novel class of small-molecule inhibitors of the MDM2-p53 interaction. Eur. J. Med. Chem. 2014, 81, 277–288. [Google Scholar] [CrossRef] [PubMed]
- OSIRIS Property Explore. Available online: http://www.organic-chemistry.Org/prog/peo/ (accessed on 1 September 2017).
- Gupta, S. Molecular steps of death receptor and mitochondrial pathways of apoptosis. Life Sci. 2001, 69, 2957–2964. [Google Scholar] [CrossRef]
- Tsai, J.H.; Hsu, L.S.; Huang, H.C.; Lin, C.L.; Pan, M.H.; Hong, H.M.; Chen, W.J. 1-(2-Hydroxy-5-methylphenyl)-3-phenyl-1,3-propanedione Induces G1 Cell Cycle Arrest and Autophagy in HeLa Cervical Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1274. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.P.; Guo, C.L.; Wang, Y.; Liu, D.; Lv, Y.B.; Lv, F.X.; Lu, Z.X. Fengycin inhibits the growth of the human lung cancer cell line 95D through reactive oxygen species production and mitochondria-dependent apoptosis. Anti-Cancer Drugs 2013, 24, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Sun, Y.; Zhou, Y.; Yin, Y.; Ding, Y.; Li, Z.; Guo, Q.; Lu, N. LYG-202 exerts antitumor effect on PI3K/Akt signaling pathway in human breast cancer cells. Apoptosis 2015, 20, 1253–1269. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.Y.; Wang, W.Y.; Luo, J.D.; Chu, S.Z.; Chen, L.J.; Xu, L.N.; Zang, H.J.; Alnemah, M.M.; Ma, J.; Fan, S.Q. CGP57380 enhances efficacy of RAD001 in non-small cell lung cancer through abrogating mTOR inhibition-induced phosphorylation of eIF4E and activating mitochondrial apoptotic pathway. Oncotarget 2016, 7, 27787–27801. [Google Scholar] [CrossRef] [PubMed]




| Compd. | n | R |
|---|---|---|
| 8a, 17a | 2 |  |
| 8b, 17b | 3 | |
| 9a, 18a | 2 |  |
| 9b, 18b | 3 | |
| 9c, 18c | 4 | |
| 10a, 19a | 2 |  |
| 10b, 19b | 3 | |
| 11a, 20a | 2 |  |
| 11b, 20b | 3 | |
| 12a, 21a | 2 |  |
| 12b, 21b | 3 | |
| 12c, 21c | 4 | |
| 13a, 22a | 2 |  |
| 13b, 22b | 3 | |
| 14a, 23a | 2 |  |
| 14b, 23b | 3 | |
| 15, 24 | 2 |  |
| 16, 25 | 2 |  |
| Compd. | CLogP a | CLogS b | TPSA (Å2) c | Ha d | Hd e | Toxicity Risks f | Drug-Likeness g |
|---|---|---|---|---|---|---|---|
| M/T/I/R | |||||||
| 17a | 4.71 | −6.79 | 105.4 | 8 | 2 | N/N/N/H | 4.44 |
| 17b | 5.16 | −7.06 | 105.4 | 8 | 2 | N/N/N/H | 3.63 |
| 18a | 3.54 | −5.90 | 114.6 | 9 | 2 | N/N/N/H | 4.80 |
| 18b | 4.00 | −6.17 | 114.6 | 9 | 2 | N/N/N/H | 4.51 |
| 18c | 4.45 | −6.44 | 114.6 | 9 | 2 | N/N/N/H | 1.00 |
| 19a | 5.21 | −6.97 | 108.6 | 9 | 2 | N/N/N/H | 5.95 |
| 19b | 5.66 | −7.24 | 108.6 | 9 | 2 | N/N/N/H | 5.96 |
| 20a | 4.89 | −6.62 | 108.6 | 9 | 2 | N/N/N/H | 8.88 |
| 20b | 5.34 | −6.89 | 108.6 | 9 | 2 | N/N/N/H | 8.91 |
| 21a | 3.65 | −5.40 | 108.6 | 9 | 2 | N/N/N/H | 9.42 |
| 21b | 4.11 | −5.67 | 108.6 | 9 | 2 | N/N/N/H | 9.84 |
| 21c | 4.56 | −5.94 | 108.6 | 9 | 2 | N/N/N/H | 6.56 |
| 22a | 3.95 | −6.51 | 134.4 | 11 | 2 | N/N/N/H | 8.98 |
| 22b | 4.41 | −6.78 | 134.4 | 11 | 2 | N/N/N/H | 9.85 |
| 23a | 3.50 | −6.18 | 134.4 | 11 | 2 | N/N/N/H | 7.58 |
| 23b | 3.95 | −6.44 | 134.4 | 11 | 2 | N/N/N/H | 8.23 |
| 24 | 2.53 | −5.42 | 145.81 | 10 | 4 | H/H/L/H | 3.32 |
| 25 | 3.35 | −4.82 | 111.83 | 10 | 2 | N/N/N/H | 9.50 |
| Compd. | IC50 (μg/mL) a | ||||
|---|---|---|---|---|---|
| A549 | Bel7402 | HepG2 | HCT116 | Caco2 | |
| TTEDM b | NA c | 44.8 ± 1.45 | NA | 31.2 ± 1.56 | 25.6 ± 0.78 |
| wlj-18 | 7.10 ± 0.53 | 9.68 ± 0.76 | 14.1 ± 1.35 | 9.78 ± 0.29 | 9.11 ± 1.23 |
| 17a | 3.15 ± 0.43 | 6.10 ± 0.78 | 4.42 ± 0.72 | 5.74 ± 0.26 | 4.23 ± 0.32 |
| 17b | 4.78 ± 0.56 | 9.98 ± 1.57 | 9.60 ± 0.34 | 3.78 ± 0.91 | 4.70 ± 0.35 |
| 18a | 10.8 ± 1.21 | 11.8 ± 1.23 | 6.63 ± 0.75 | 6.22 ± 0.23 | 5.61 ± 0.89 |
| 18b | 7.62 ± 0.76 | 48.6 ± 3.23 | 25.0 ± 1.66 | 16.8 ± 0.65 | 10.2 ± 1.28 |
| 18c | 4.49 ± 0.73 | NA | 42.4 ± 1.86 | NA | NA |
| 19a | 12.6 ± 0.98 | 10.9 ± 0.77 | 8.79 ± 0.65 | 4.89 ± 0.35 | 5.76 ± 0.82 |
| 19b | 3.60 ± 0.38 | 6.78 ± 0.76 | 5.42 ± 0.92 | 3.82 ± 0.43 | 1.70 ± 0.24 |
| 20a | 8.10 ± 1.11 | 9.40 ± 0.88 | 8.42 ± 0.76 | 5.07 ± 0.69 | 5.94 ± 1.32 |
| 20b | 6.99 ± 1.42 | 8.67 ± 0.37 | 7.83 ± 1.56 | 5.80 ± 0.21 | 8.24 ± 1.42 |
| 21a | 11.5 ± 0.27 | 15.1 ± 1.87 | 8.67 ± 0.53 | 5.37 ± 0.79 | 6.89 ± 0.55 |
| 21b | 5.20 ± 0.76 | 3.25 ± 0.32 | 5.83 ± 1.11 | 4.43 ± 0.53 | 7.52 ± 0.99 |
| 21c | 16.7 ± 3.39 | NA | 40.7 ± 2.13 | 43.3 ± 0.23 | 41.9 ± 3.89 |
| 22a | 7.40 ± 0.46 | 17.3 ± 1.98 | 6.80 ± 1.11 | 3.59 ± 0.25 | 4.09 ± 0.76 |
| 22b | 7.10 ± 1.02 | 44.8 ± 2.44 | 41.1 ± 3.12 | 34.2 ± 2.23 | 5.60 ± 0.46 |
| 23a | 14.9 ± 3.26 | 33.7 ± 2.22 | 21.6 ± 1.73 | 8.90 ± 0.43 | 8.50 ± 1.81 |
| 23b | 5.96 ± 1.02 | NA | NA | 22.3 ± 2.18 | 8.97 ± 0.29 |
| 24 | 29.9 ± 2.72 | 32.7 ± 0.24 | NA | 15.0 ± 1.36 | 22.7 ± 2.11 |
| 25 | 11.5 ± 2.41 | 12.9 ± 1.38 | 14.9 ± 0.87 | 10.1 ± 0.81 | 9.3 ± 1.43 |
| Sunitinib d | 11.6 ± 1.24 | 4.50 ± 0.58 | 5.80 ± 1.08 | 3.70 ± 0.27 | 2.7 ± 0.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-J.; Guo, C.-L.; Li, X.-Q.; Wang, S.-Y.; Jiang, B.; Zhao, Y.; Luo, J.; Xu, K.; Liu, H.; Guo, S.-J.; et al. Discovery of Novel Bromophenol Hybrids as Potential Anticancer Agents through the Ros-Mediated Apoptotic Pathway: Design, Synthesis and Biological Evaluation. Mar. Drugs 2017, 15, 343. https://doi.org/10.3390/md15110343
Wang L-J, Guo C-L, Li X-Q, Wang S-Y, Jiang B, Zhao Y, Luo J, Xu K, Liu H, Guo S-J, et al. Discovery of Novel Bromophenol Hybrids as Potential Anticancer Agents through the Ros-Mediated Apoptotic Pathway: Design, Synthesis and Biological Evaluation. Marine Drugs. 2017; 15(11):343. https://doi.org/10.3390/md15110343
Chicago/Turabian StyleWang, Li-Jun, Chuan-Long Guo, Xiang-Qian Li, Shuai-Yu Wang, Bo Jiang, Yue Zhao, Jiao Luo, Kuo Xu, Hua Liu, Shu-Ju Guo, and et al. 2017. "Discovery of Novel Bromophenol Hybrids as Potential Anticancer Agents through the Ros-Mediated Apoptotic Pathway: Design, Synthesis and Biological Evaluation" Marine Drugs 15, no. 11: 343. https://doi.org/10.3390/md15110343