The Bacterial (Vibrio alginolyticus) Production of Tetrodotoxin in the Ribbon Worm Lineus longissimus—Just a False Positive?
Abstract
:1. Introduction
2. Results and Discussion
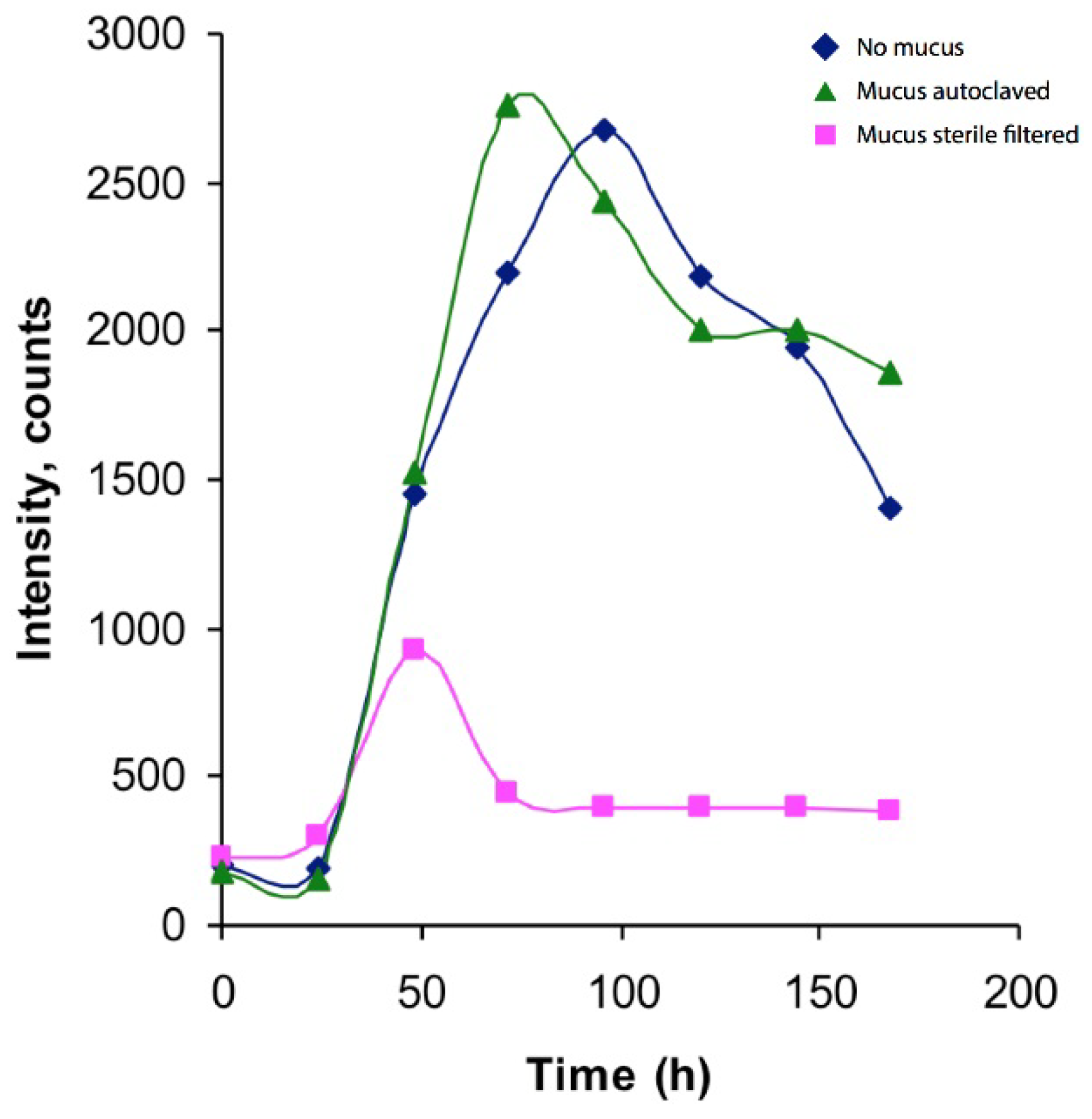
2.1. Establishment and Cultivation of Vibrio Alginolyticus
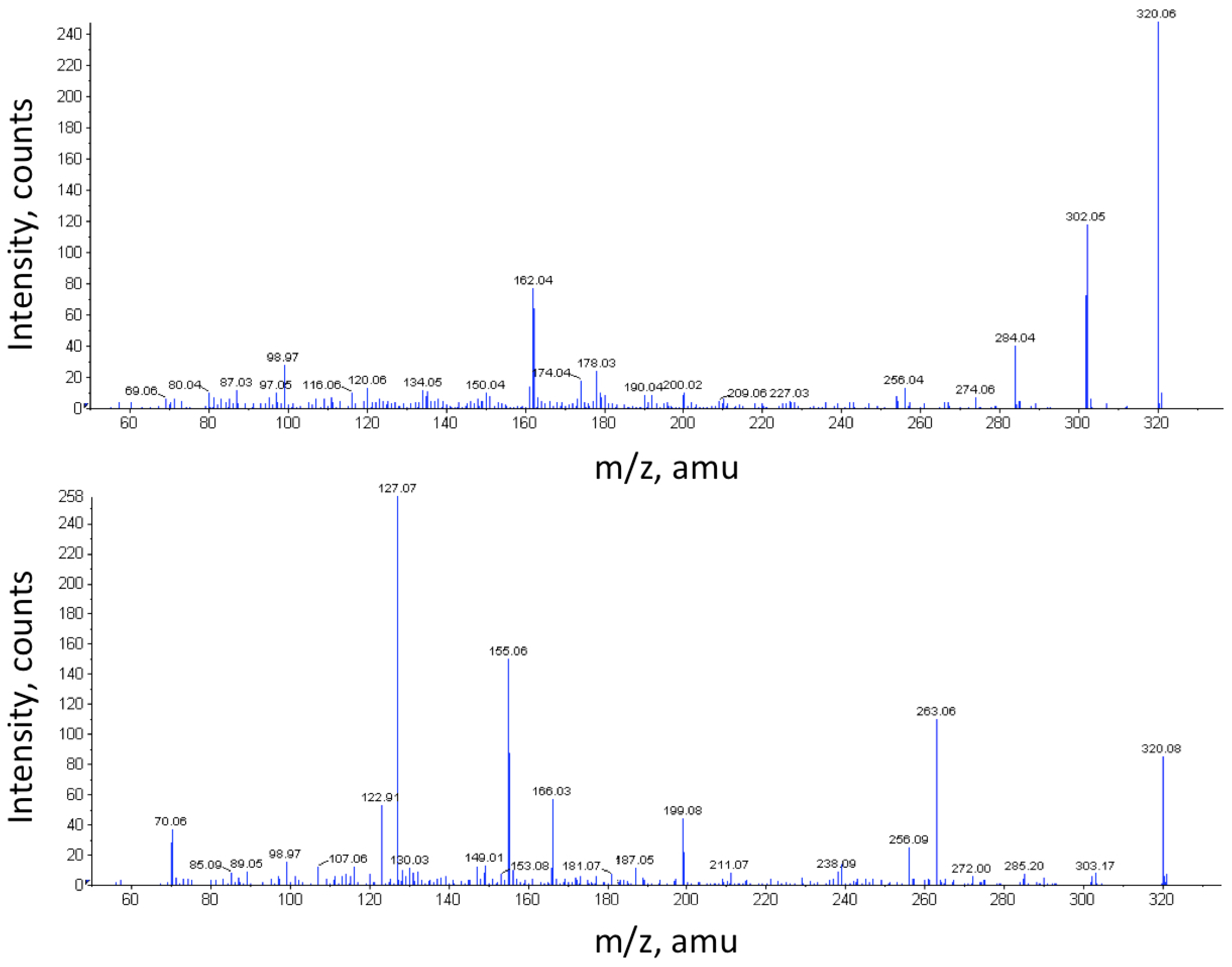
2.2. Chemical Analyses for the Detection of TTX
3. Experimental Section
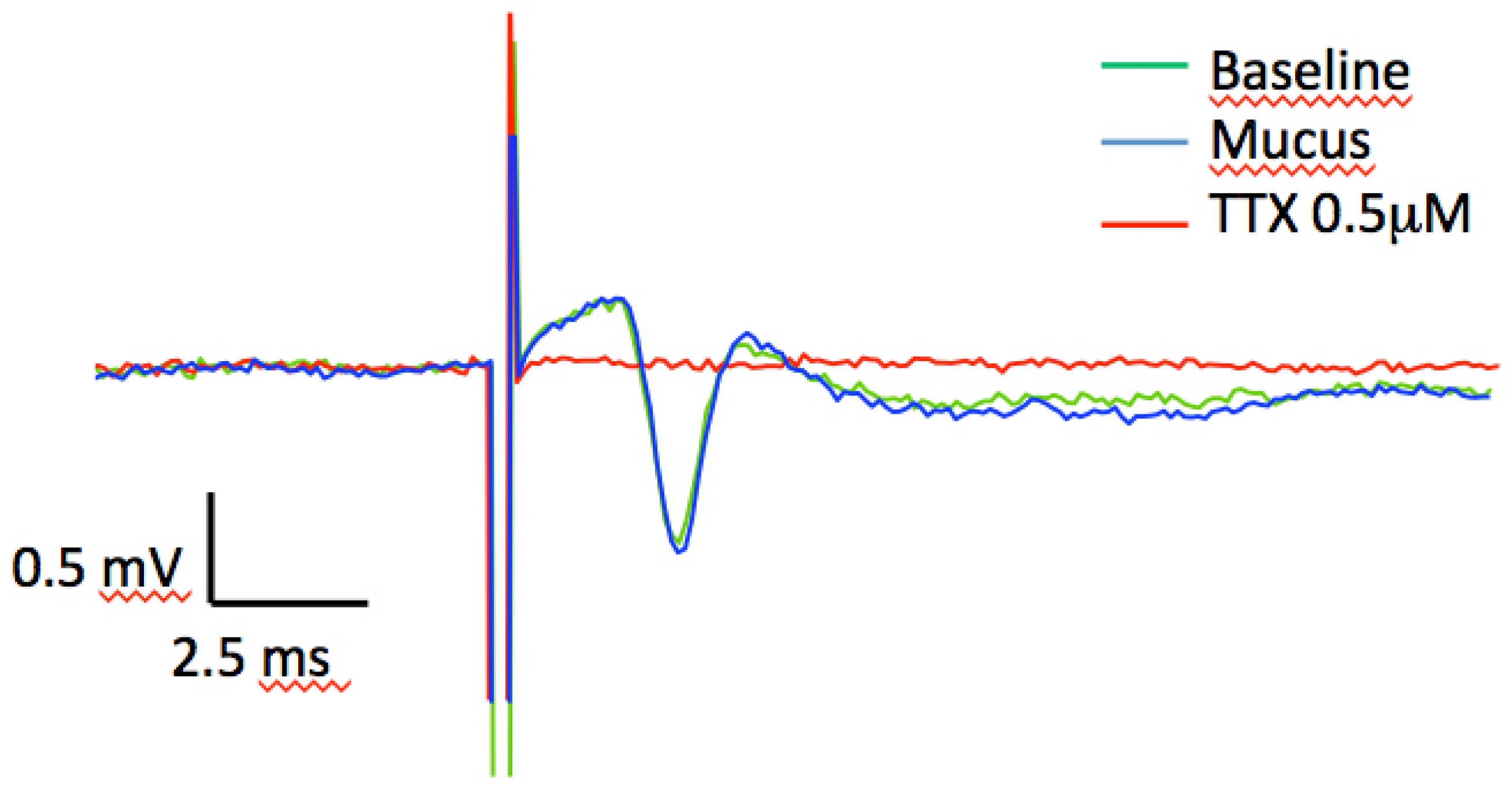
3.1. L. longissimus Mucus Collection
3.2. Shore Crab Bioassay of TTX and L. longissimus Mucus
3.3. Bacterial Growth and Identification
3.4. LC and LC-MS on V. alginolyticus Cultures
3.5. Attempted TTX-Enrichment from Mucus of L. longissimus
3.6. Analysis of Potential TTX-Macromolecular Association
3.7. Boronate-Affinity Chromatography of L. longissimus Mucus
3.8. Size Fractionation of L. longissimus Mucus
3.9. Preparation of Rat Hippocampal Slices
3.10. Axonal Conductance Recording and Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bane, V.; Lehane, M.; Dikshit, M.; O‘Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Ruben, P.C. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels 2008, 2, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Gellens, M.E.; George, A.L., Jr.; Chen, L.Q.; Chahine, M.; Horn, R.; Barchi, R.L.; Kallen, R.G. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc. Natl. Acad. Sci. USA 1992, 89, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.N.; Sivilotti, L.; Wood, J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996, 379, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O. Tetrodotoxin—Distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef] [PubMed]
- Kem, W.R. Structure and activity of nemertine toxins. Integr. Comp. Biol. 1985, 25, 99–111. [Google Scholar]
- Miyazawa, K.; Higashiyama, M.; Ito, K.; Noguchi, T.; Arakawa, O.; Shida, Y.; Hashimoto, K. Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon 1988, 26, 867–874. [Google Scholar] [CrossRef]
- Carroll, S.; McEvoy, E.G.; Gibson, R. The production of tetrodotoxin-like substances by nemertean worms in conjunction with bacteria. J. Exp. Mar. Biol. Ecol. 2003, 288, 51–63. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Shida, Y.; Noguchi, T.; Miyazawa, K. Paralytic toxins in a ribbon worm Cephalothrix species (Nemertean) adherent to cultured oysters in Hiroshima bay, Hiroshima prefecture, Japan. Toxicon 2000, 38, 763–773. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Ito, K.; Bessho, K.; Yamaguchi, C.; Tsunetsugu, S.; Shida, Y.; Kajihara, H.; Mawatari, S.F.; Noguchi, T.; et al. Paralytic toxicity in the ribbon worm Cephalothrix species (Nemertea) in Hiroshima bay, Hiroshima prefecture, Japan, and the isolation of tetrodotoxin as a main component of its toxins. Toxicon 2003, 41, 747–753. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Arakawa, O.; Takatani, T.; Kajihara, H.; Kawatsu, K.; Hamano, Y.; Asakawa, M.; Miyazawa, K.; Noguchi, T. Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (Nemertea: Anopla: Palaeonemertea: Cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae). Toxicon 2004, 44, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093. [Google Scholar] [CrossRef]
- Noguchi, T.; Ali, A.E.; Arakawa, O.; Miyazawa, K.; Kanoh, S.; Shida, Y.; Nishio, S.; Hashimoto, K. Tetrodonic acid-like substance; a possible precursor of tetrodotoxin. Toxicon 1991, 29, 845–855. [Google Scholar] [CrossRef]
- Asakawa, M.; Ito, K.; Kajihara, H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima bay, Hiroshima prefecture, Japan. Toxins 2013, 5, 376–395. [Google Scholar] [CrossRef] [PubMed]
- Magarlamov, T.Y.; Beleneva, I.A.; Chernyshev, A.V.; Kuhlevsky, A.D. Tetrodotoxin-producing Bacillus sp. from the ribbon worm (Nemertea) Cephalothrix simula (Iwata, 1952). Toxicon 2014, 85, 46–51. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, E.G.; Rogers, A.; Gibson, R. Preliminary investigation of Vibrio alginolyticus-like bacteria associated with marine nemerteans. Hydrobiologia 1998, 365, 287–291. [Google Scholar] [CrossRef]
- Dorsch, M.; Lane, D.; Stackebrandt, E. Towards a phylogeny of the genus Vibrio based on 16s rRNA sequences. Int. J. Syst. Evol. Microbiol. 1992, 42, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-F.; Yu, P.H.-F.; Chan, P.-L.; Yan, Q.; Wong, P.-K. Two novel species of tetrodotoxin-producing bacteria isolated from toxic marine puffer fishes. Toxicon 2004, 44, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, E.V.; Kogure, K. Bacterial production of tetrodotoxin in 4 species of Chaetognatha. Biol. Bull. 1989, 176, 191–194. [Google Scholar] [CrossRef]
- Jang, J.-H.; Lee, J.-S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K. Reexamination of tetrodotoxin production by bacteria. Appl. Environ. Microbiol. 1995, 61, 3468–3470. [Google Scholar] [PubMed]
- Matsumoto, T.; Tanuma, D.; Tsutsumi, K.; Jeon, J.K.; Ishizaki, S.; Nagashima, Y. Plasma protein binding of tetrodotoxin in the marine puffer fish Takifugu rubripes. Toxicon 2010, 55, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T.; Haas, H.G.; Therrien, E.F. Saxitoxin and tetrodotoxin: Comparison of nerve blocking mechanism. Science 1967, 157, 1441–1442. [Google Scholar] [CrossRef] [PubMed]
- Kem, W.R. Biochemistry of nemertine toxins. In Marine Pharmacognosy. Action of Marine Biotoxins at the Cellular Level; Martin, D., Padilla, G., Eds.; Academic Press: New York, NY, USA, 1973; pp. 38–85. [Google Scholar]
- Pfeffer, C.; Oliver, J.D. A comparison of thiosulphate-citrate-bile salts-sucrose (TCBS) agar and thiosulphate-chloride-iodide (TCI) agar for the isolation of Vibrio species from estuarine environments. Lett. Appl. Microbiol. 2003, 36, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Riebe, I.; Hanse, E. Development of synaptic connectivity onto interneurons in stratum radiatum in the CA1 region of the rat hippocampus. BMC Neurosci. 2012, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narita, H.; Matsubara, S.; Miwa, N.; Akahane, S.; Murakami, M.; Goto, T.; Nara, M.; Noguchi, T.; Saito, T.; Shida, Y.; et al. Vibrio alginolyticus, a TTX-producing bacterium isolated from the starfish Astropecten polyacanthus. Bull. Jpn. Soc. Sci. Fish. 1987, 53, 617–621. [Google Scholar] [CrossRef]
- Noguchi, T.; Jeon, J.K.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of a xanthid crab, Atergatis floridus. J. Biochem. 1986, 99, 311–314. [Google Scholar] [PubMed]
- Ritchie, K.B.; Nagelkerken, I.; James, S.; Smith, G.W. A tetrodotoxin-producing marine pathogen. Nature 2000, 404, 354. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jeong, D.Y.; Kim, W.S.; Kim, H.D.; Kim, C.H.; Park, W.W.; Park, Y.H.; Kim, K.S.; Kim, H.M.; Kim, D.S. A tetrodotoxin-producing Vibrio strain, LM-1, from the puffer fish Fugu vermicularis radiatus. Appl. Environ. Microbiol. 2000, 66, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Harada, R.M.; DeFelice, S.V.; Bienfang, P.K.; Li, Q.X. Bacterial production of tetrodotoxin in the pufferfish Arothron hispidus. Nat. Prod. Res. 2009, 23, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Kem, W.R. Purification and characterization of a new family of polypeptide neurotoxins from the heteronemertine Cerebratulus lacteus (leidy). J. Biol. Chem. 1976, 251, 4184–4192. [Google Scholar] [PubMed]
- Whelan, N.V.; Kocot, K.M.; Santos, S.R.; Halanych, K.M. Nemertean toxin genes revealed through transcriptome sequencing. Genome Biol. Evol. 2014, 6, 3314–3325. [Google Scholar] [CrossRef] [PubMed]
- Salvitti, L.; Wood, S.; McNabb, P.; Cary, S. No evidence for a culturable bacterial tetrodotoxin producer in Pleurobranchaea maculata (gastropoda: Pleurobranchidae) and Stylochoplana sp. (platyhelminthes: Polycladida). Toxins 2015, 7, 255–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Class | Order | Species | Source |
|---|---|---|---|
| Anopla | Palaeonemertea | Cephalothrix sp. | [11,12,13] |
| Cephalothrix linearis | [14,15] | ||
| Cephalothrix rufifrons | [10] | ||
| Cephalothrix simula | [16,17] | ||
| Tubulanus punctatus | [9] | ||
| Heteronemertea | Lineus alborostratus | [16] | |
| Lineus fuscoviridis | [9] | ||
| Lineus longissimus | [10] | ||
| Lineus ruber | [10] | ||
| Lineus torquatus | [16] | ||
| Lineus viridis | [10] | ||
| Ramphogordius sanguineus | [10] | ||
| Riseriellus occultus | [10] | ||
| Enopla | Hoplonemertea | Amphiporus sp. | [16] |
| Amphiporus lactifloreus | [10] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strand, M.; Hedström, M.; Seth, H.; McEvoy, E.G.; Jacobsson, E.; Göransson, U.; Andersson, H.S.; Sundberg, P. The Bacterial (Vibrio alginolyticus) Production of Tetrodotoxin in the Ribbon Worm Lineus longissimus—Just a False Positive? Mar. Drugs 2016, 14, 63. https://doi.org/10.3390/md14040063
Strand M, Hedström M, Seth H, McEvoy EG, Jacobsson E, Göransson U, Andersson HS, Sundberg P. The Bacterial (Vibrio alginolyticus) Production of Tetrodotoxin in the Ribbon Worm Lineus longissimus—Just a False Positive? Marine Drugs. 2016; 14(4):63. https://doi.org/10.3390/md14040063
Chicago/Turabian StyleStrand, Malin, Martin Hedström, Henrik Seth, Eric G. McEvoy, Erik Jacobsson, Ulf Göransson, Håkan S. Andersson, and Per Sundberg. 2016. "The Bacterial (Vibrio alginolyticus) Production of Tetrodotoxin in the Ribbon Worm Lineus longissimus—Just a False Positive?" Marine Drugs 14, no. 4: 63. https://doi.org/10.3390/md14040063