Antimicrobial and Antitumor Activities of Novel Peptides Derived from the Lipopolysaccharide- and β-1,3-Glucan Binding Protein of the Pacific Abalone Haliotis discus hannai
Abstract
:1. Introduction
2. Results
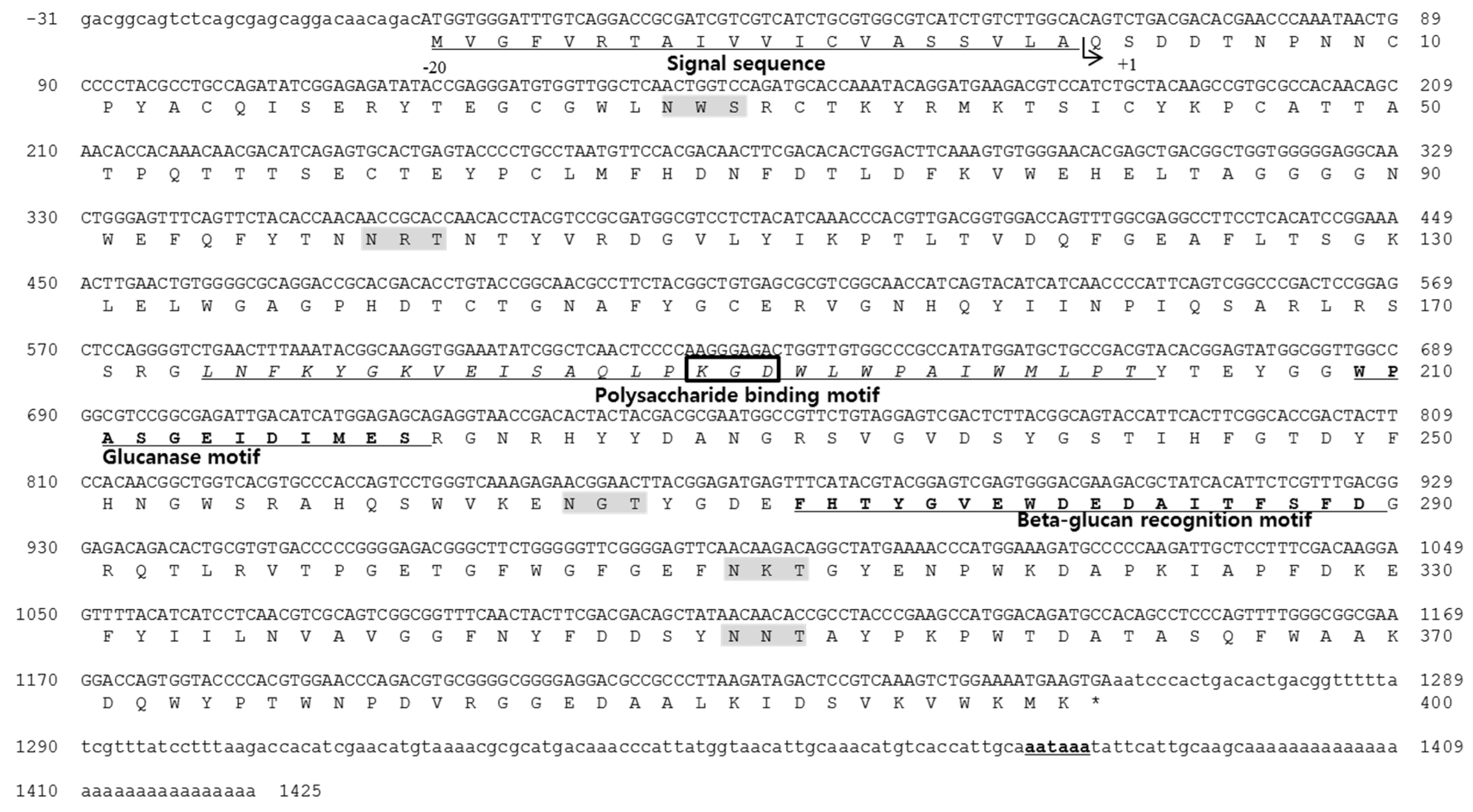
2.1. Identification of the Antimicrobial Peptide and cDNA Sequences
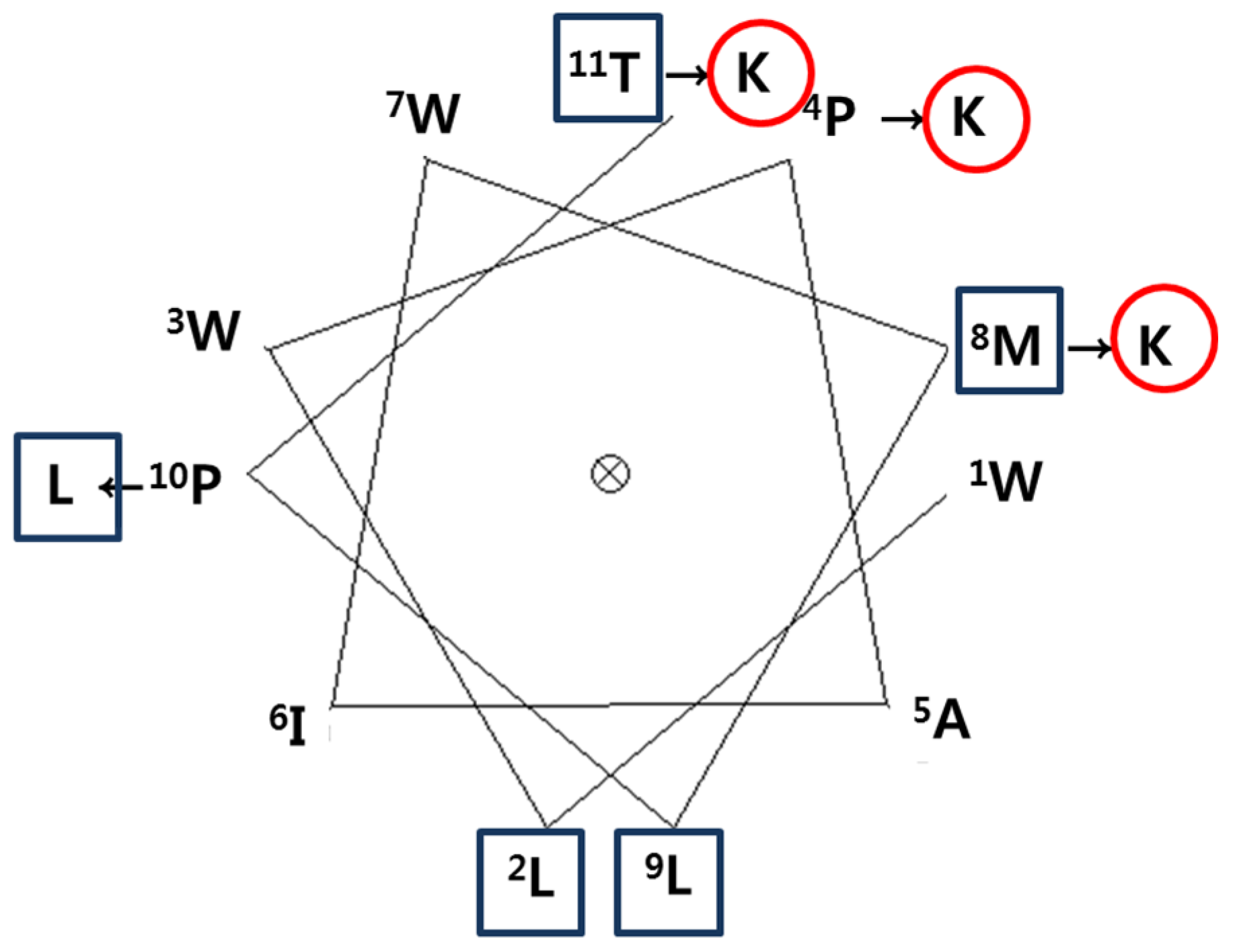
2.2. Peptide Design and Synthesis
2.3. Antimicrobial Activity of HDH-LGBP Analogs
2.4. Thermal Stability of HDH-LGBP Analogs
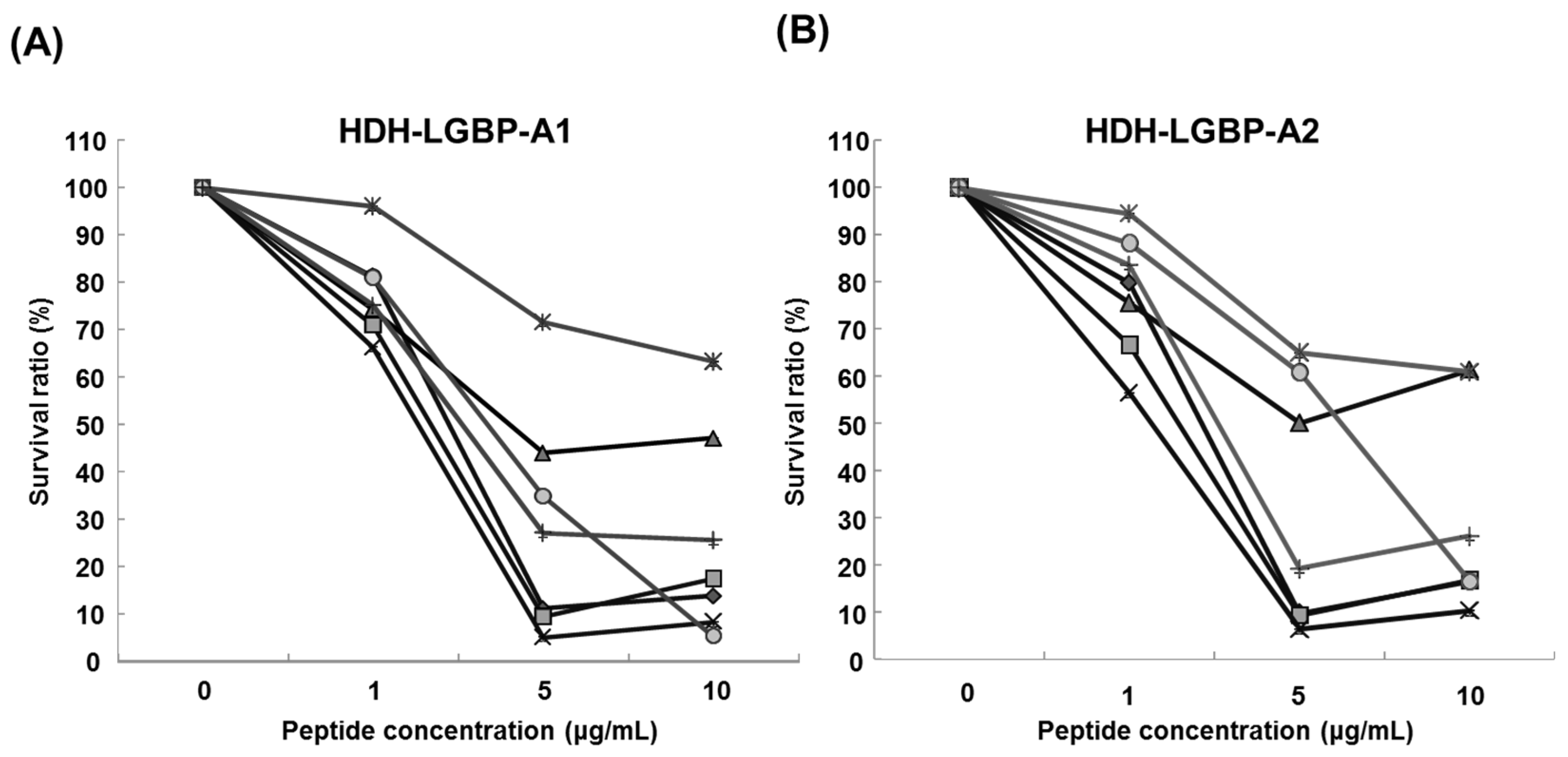
2.5. Cytotoxicity of HDH-LGBP Analogs
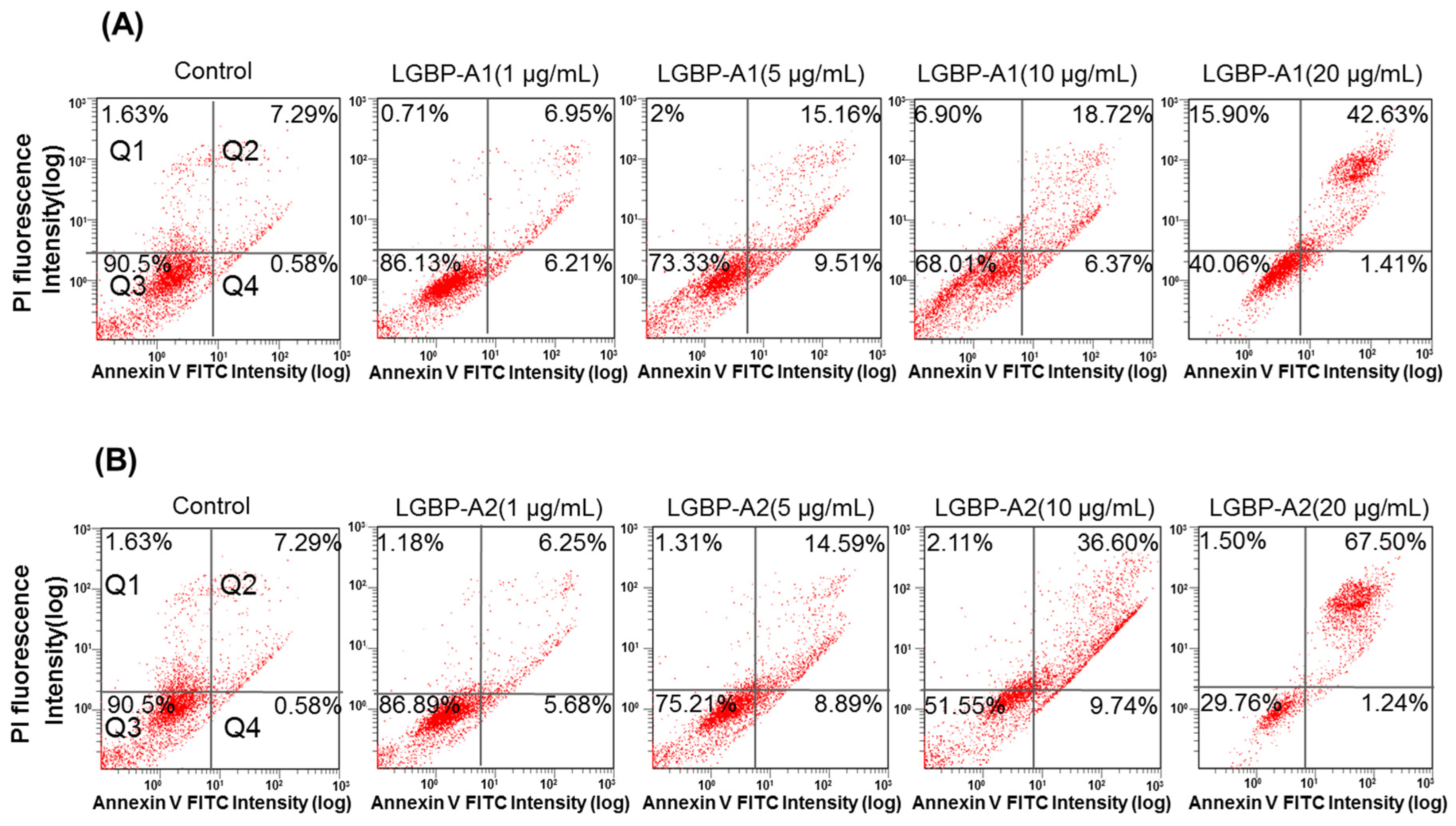
2.6. Effect of HDH-LGBP on Cancer Cell Membranes
3. Discussion
4. Materials and Methods
4.1. Cloning and Sequencing the Full-Length cDNA of Abalone LGBP
4.2. Computational Sequence Analysis
4.3. Structure Prediction
4.4. Peptide Design and Synthesis
4.5. Ultrasensitive Radial Diffusion Assay (URDA) for Antimicrobial Potency
4.6. Minimal Effective Concentration of the GBP-Derived Analogs
4.7. Effect of Temperature on Antimicrobial Activity
4.8. Cell Culture
4.9. Cell Viability
4.10. FITC-Annexin V and Propidium Iodide (PI) Staining
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoffman, O.A.; Olson, E.J.; Limper, A.H. Fungal beta glucans modulate macrophage release of tumor necrosis factor-alpha in response to bacterial lipopolysaccharide. Immunol. Lett. 1993, 37, 19–25. [Google Scholar] [CrossRef]
- Lee, S.Y.; Wang, R.; Söderhäll, K. A lipopolysaccharide- and beta-1,3-glucan-binding protein from hemocytes of the freshwater crayfish Pacifastacus leniusculus. Purification, characterization, and cDNA cloning. J. Biol. Chem. 2000, 275, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Vaseeharan, B.; Chen, J.C. Identification and phylogenetic analysis on lipopolysaccharide and beta-1,3-glucan binding protein (LGBP) of kuruma shrimp Marsupenaeus japonicus. Dev. Comp. Immunol. 2008, 32, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, F.; Dong, B.; Wang, X.; Xiang, J. Molecular cloning and characterisation of a pattern recognition protein, lipopolysaccharide and beta-1,3-glucan binding protein (LGBP) from Chinese shrimp Fenneropenaeus chinensis. Mol. Biol. Rep. 2009, 36, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Ni, D.; Song, L.; Zhao, J.; Qiu, L. Molecular cloning and characterization of a short type peptidoglycan recognition protein (CfPGRP-S1) cDNA from Zhikong scallop Chlamys farreri. Fish Shellfish Immunol. 2007, 23, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Nikapitiya, C.; De Zoysa, M.; Lee, J. Molecular characterization and gene expression analysis of a pattern recognition protein from disk abalone, Haliotis discus discus. Fish Shellfish Immunol. 2008, 25, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, J.; Jiang, J.; Qiu, L.; Zhu, C.; Su, T.; Li, Y.; Wu, K.; Jiang, S. Molecular characterization and expression analysis of lipopolysaccharide and β-1,3-glucan-binding protein (LGBP) from pearl oyster Pinctada fucata. Mol. Biol. Rep. 2010, 37, 3335–3343. [Google Scholar] [CrossRef] [PubMed]
- Imjongjirak, C.; Amparyup, P.; Tassanakajon, A.; Sittipraneed, S. Antilipopolysaccharide factor (ALF) of mud crab Scylla paramamosain: Molecular cloning, genomic organization and the antimicrobial activity of its synthetic LPS binding domain. Mol. Immunol. 2007, 44, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Chao, T.T.; Chen, J.C.; Chen, J.Y.; Liu, W.C.; Lin, C.H.; Kuo, C.M. Shrimp (Penaeus monodon) anti-lipopolysaccharide factor reduces the lethality of Pseudomonas aeruginosa sepsis in mice. Int. Immunopharmacol. 2007, 7, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Yedery, R.D.; Patgaonkar, M.S.; Selvaakumar, C.; Reddy, K.V. Antibacterial activity of a synthetic peptide that mimics the LPS binding domain of Indian mud crab, Scylla serrata anti-lipopolysaccharide factor (SsALF) also involved in the modulation of vaginal immune functions through NF-kB signaling. Microb. Pathog. 2011, 50, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Y.; Li, S.H.; Li, F.H.; Zhang, X.J.; Xiang, J.H. Modification of a synthetic LPS-binding domain of anti-lipopolysaccharide factor from shrimp reveals strong structure-activity relationship in their antimicrobial characteristics. Dev. Comp. Immunol. 2014, 45, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Guo, S.Y.; Li, F.H.; Xiang, J.H. Characterization and function analysis of an anti-lipopolysaccharide factor (ALF) from the Chinese shrimp Fenneropenaeus chinensis. Dev. Comp. Immunol. 2014, 46, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Guo, S.Y.; Li, F.H.; Xiang, J.H. Functional diversity of anti-lipopolysaccharide factor isoforms in shrimp and their characters related to antiviral activity. Mar. Drugs 2015, 13, 2602–2616. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Luna, J.S.; Campos-Rodriguez, R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kwon, H.M.; Park, J.W.; Kurokawa, K.; Lee, B.L. N-terminal GNBP homology domain of Gram-negative binding protein 3 functions as a beta-1,3-gluganc binding motif in Tenebrio molitor. BMB Rep. 2009, 42, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. From antimicrobial to cytotoxic peptides. A review. Front. Microbiol. 2013, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ausbacher, D.; Svineng, G.; Hansen, T.; Strom, M.B. Cytotoxic mechanisms of action of two small amphipathic beta(2,2)-amino acid derivatives derived from antimicrobial peptides. Biochim. Biophys. Acta 2012, 1818, 2917–2925. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Oren, Z.; Hong, J.; Shai, Y. A comparative study on the structure and function of a cytolytic alpha-helical peptide and its antimicrobial beta-sheet diastereomer. Eur. J. Biochem. 1999, 259, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.D.; Vergnes, A.; de Lorgeril, J.; Goncalves, P.; Perazzolo, L.M.; Sauné, L.; Romestand, B.; Fievet, J.; Gueguen, Y.; Bachère, E.; et al. Functional divergence in shrimp anti-lipopolysaccharide factors (ALFs): From recognition of cell wall components to antimicrobial activity. PLoS ONE 2013, 8, e67937. [Google Scholar] [CrossRef] [PubMed]
- Tharntada, S.; Ponprateep, S.; Somboonwiwat, K.; Liu, H.; Söderhäll, I.; Söderhäll, K.; Tassanakajon, A. Role of anti-lipopolysaccharide factor from the black tiger shrimp, Penaeus monodon, in protection from white spot syndrome virus infection. J. Gen. Virol. 2009, 90, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Pouny, Y.; Shai, Y. Interaction of d-amino acid incorporated analogs of pardaxin with membranes. Biochemistry 1992, 31, 9482–9490. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on cytotoxic activities of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human b-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef]
- Park, E.M.; Nam, B.H.; Kim, Y.O.; Kong, H.J.; Kim, W.J.; Lee, S.J.; Kong, I.S.; Choi, T.J. EST-based survey of gene expression in seven tissue types from the abalone Haliotis discus hannai. J. Fish. Sci. Technol. 2007, 10, 119–126. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: http://ncbi.nlm.nih.gov/blast/ (accessed on 8 January 2013).
- SignalP 4.1 Server. Available online: http://www.cbs.dtu.dk/services/SignalP/ (accessed on 11 April 2013).
- Pfam Protein Family Database. Available online: http://pfam.xfam.org/search/ (accessed on 30 June 2013).
- SIB Bioinformatics Resources Portal. Available online: http://web.expasy.org/peptide_mass/ (accessed on 30 June 2013).
- Ramachandran, G.N.; Sasisekharan, V. Conformation of polypeptides and proteins. Adv. Prot. Chem. 1968, 23, 283–437. [Google Scholar]
- Boman, H. Antibacterial peptides, basic facts and emerging concepts. J. Int. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef]
- The Antimicrobial Peptide Database. Available online: http://aps.unmc.edu/Ap/main.php/ (accessed on 30 June 2013).
- Seo, J.K.; Crawford, J.M.; Stone, K.L.; Noga, E.J. Purification of a novel arthropod defensin from the American oyster, Crassostrea virginica. Biochem. Biophys. Res. Commun. 2005, 338, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Rosenman, M.; Harwig, S.S.L.; Jackson, R.; Eisenhaur, P. Ultrasensitive assay for endogenous antimicrobial polypeptides. J. Immunol. Methods 1991, 137, 167–173. [Google Scholar] [CrossRef]

| Peptide Name | Sequence | Length | M.W. | p.I. | Hydrophobicity | Hydrophobicmoment | Charge | Boman Index (kcal/mol) | Structure |
|---|---|---|---|---|---|---|---|---|---|
| HDH-LGBP-N | WLWPAIWMLPT-OH | 11 | 1413.7 | 5.52 | −1.62 | 0.11 | 0 | −2.12 | T & R |
| HDH-LGBP-A1 | WLWKAIWKLLT-NH2 | 11 | 1457.8 | 10.0 | −1.12 | 0.86 | +3 | −1.34 | H |
| HDH-LGBP-A2 | WLWKAIWKLLK-NH2 | 11 | 1484.8 | 10.3 | −0.81 | 1.07 | +4 | −1.07 | H |
| Microbe | Minimal Effective Concentration (μg/mL) | ||
|---|---|---|---|
| Gram | HDH-LGBP-A1 | HDH-LGBP-A2 | |
| B. cereus | + | 1.9 | 1.8 |
| S. aureus RM4220 | + | 1.08 | 1.37 |
| S. iniae FP5229 | + | 0.57 | 1.79 |
| S. mutans | + | 0.008 | 1.7 |
| P. aeruginosa KCTC2004 | − | 2.12 | 1.92 |
| V. anguillarum | − | >125 | >125 |
| V. harveyi | − | >125 | >125 |
| C. albicans KCTC7965 | Yeast | 2.11 | 2.16 |
| Peptide Name | Microbe | S. aureus | P. aeroginosa | C. albicans |
|---|---|---|---|---|
| HDH-LGBP-A1 | N |  |  |  |
| H |  |  |  | |
| HDH-LGBP-A2 | N |  |  |  |
| H |  |  |  |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, B.-H.; Moon, J.Y.; Park, E.H.; Kong, H.J.; Kim, Y.-O.; Kim, D.-G.; Kim, W.-J.; An, C.M.; Seo, J.-K. Antimicrobial and Antitumor Activities of Novel Peptides Derived from the Lipopolysaccharide- and β-1,3-Glucan Binding Protein of the Pacific Abalone Haliotis discus hannai. Mar. Drugs 2016, 14, 227. https://doi.org/10.3390/md14120227
Nam B-H, Moon JY, Park EH, Kong HJ, Kim Y-O, Kim D-G, Kim W-J, An CM, Seo J-K. Antimicrobial and Antitumor Activities of Novel Peptides Derived from the Lipopolysaccharide- and β-1,3-Glucan Binding Protein of the Pacific Abalone Haliotis discus hannai. Marine Drugs. 2016; 14(12):227. https://doi.org/10.3390/md14120227
Chicago/Turabian StyleNam, Bo-Hye, Ji Young Moon, Eun Hee Park, Hee Jeong Kong, Young-Ok Kim, Dong-Gyun Kim, Woo-Jin Kim, Chul Min An, and Jung-Kil Seo. 2016. "Antimicrobial and Antitumor Activities of Novel Peptides Derived from the Lipopolysaccharide- and β-1,3-Glucan Binding Protein of the Pacific Abalone Haliotis discus hannai" Marine Drugs 14, no. 12: 227. https://doi.org/10.3390/md14120227
APA StyleNam, B.-H., Moon, J. Y., Park, E. H., Kong, H. J., Kim, Y.-O., Kim, D.-G., Kim, W.-J., An, C. M., & Seo, J.-K. (2016). Antimicrobial and Antitumor Activities of Novel Peptides Derived from the Lipopolysaccharide- and β-1,3-Glucan Binding Protein of the Pacific Abalone Haliotis discus hannai. Marine Drugs, 14(12), 227. https://doi.org/10.3390/md14120227





