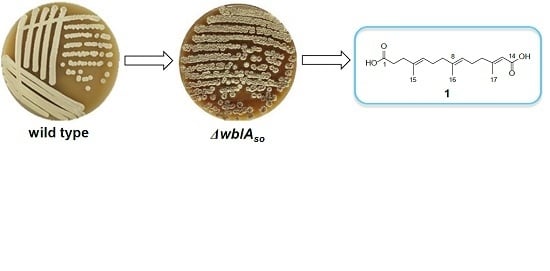
A New Dioic Acid from a wbl Gene Mutant of Deepsea-Derived Streptomyces somaliensis SCSIO ZH66
Abstract
:1. Introduction
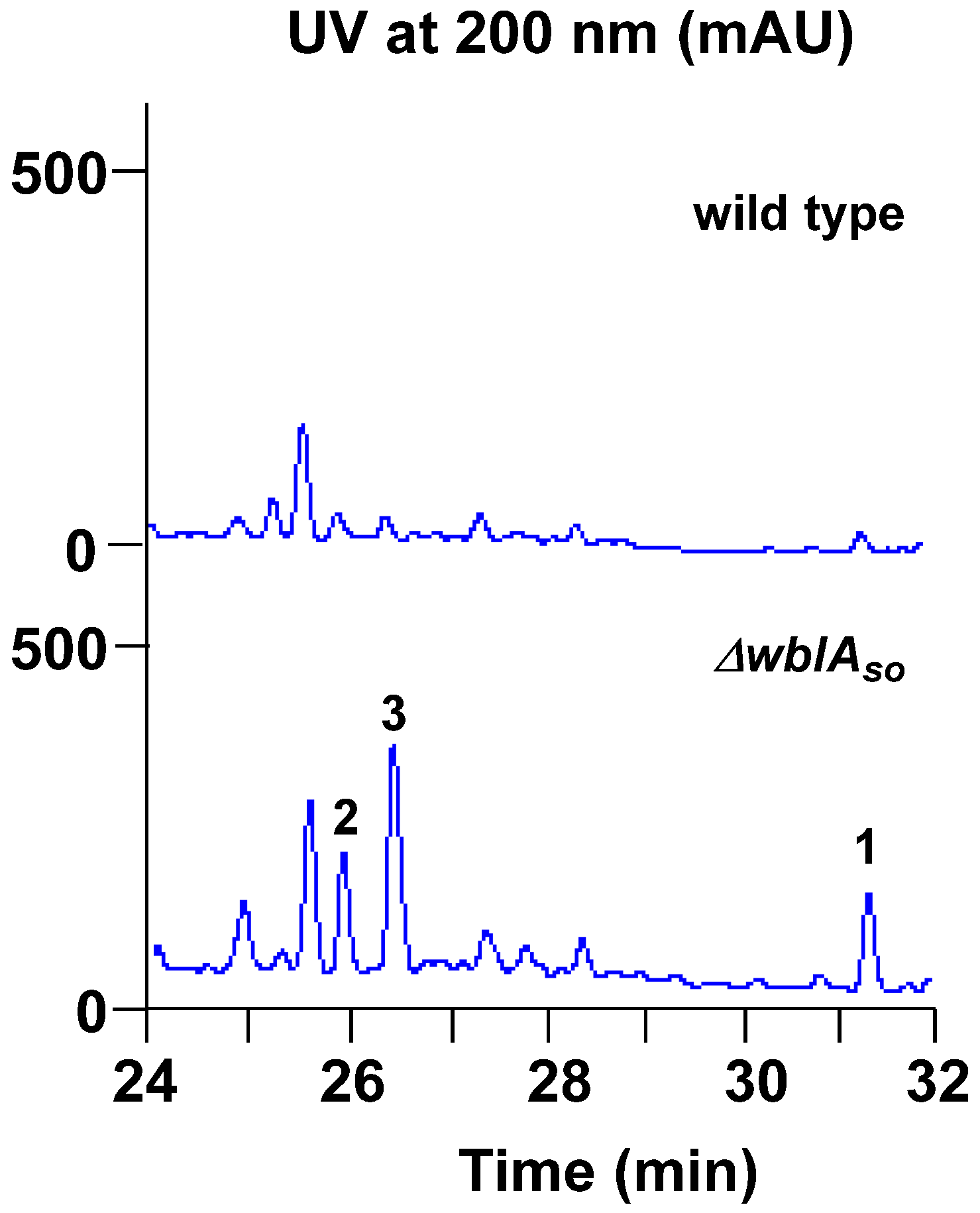
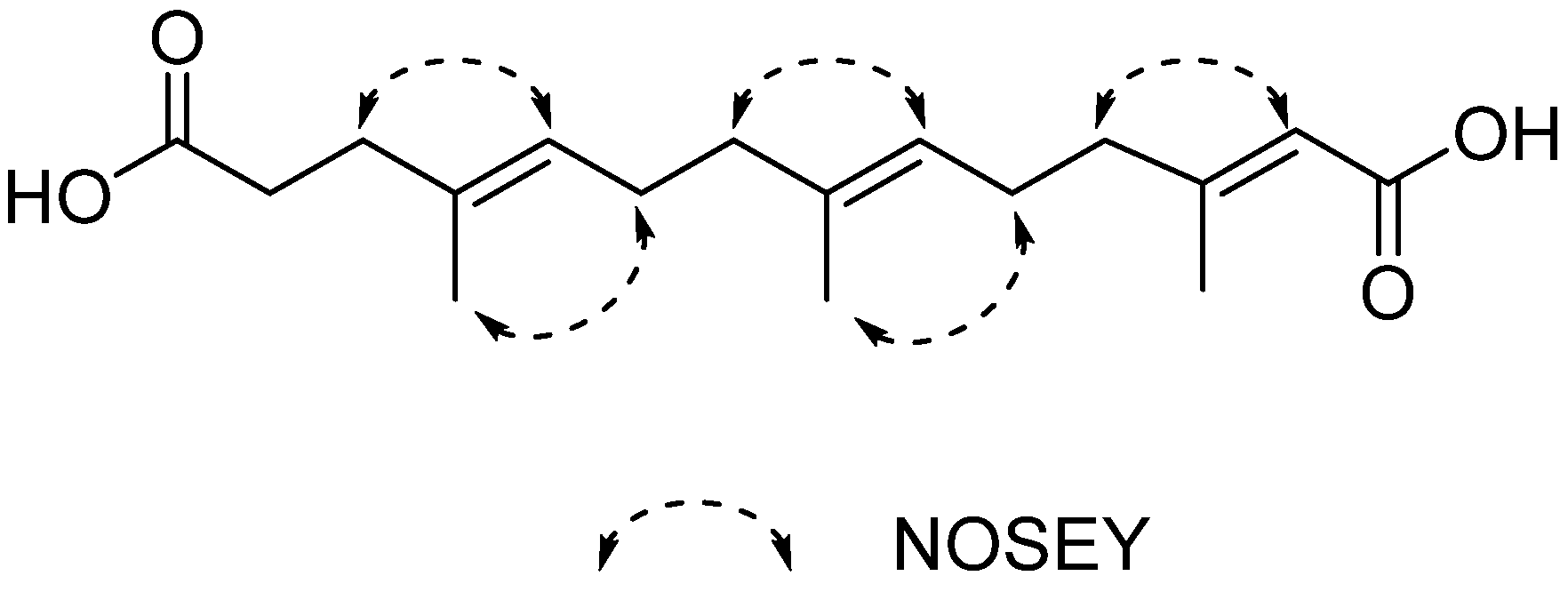
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Strains and Culture Conditions
3.3. Fermentation, Extraction, and Isolation of the Compounds
3.4. Biological Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Gupta, L.; Talwar, A.; Chauhan, P.M. Bis and tris indole alkaloids from marine organisms: New leads for drug discovery. Curr. Med. Chem. 2007, 14, 1789–1803. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.L.; Moore, B.S. A sea of biosynthesis: Marine natural products meet the molecular age. Nat. Prod. Rep. 2011, 28, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Zotchev, S.B. Marine actinomycetes as an emerging resource for the drug development pipelines. J. Biotechnol. 2012, 158, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L.; Marti, G.; Ferreira, Q.E. Advances in techniques for profiling crude extracts and for the rapid identification of natural products: Dereplication, quality control and metabolomics. Curr. Org. Chem. 2010, 14, 1808–1832. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Wray, V.; Lai, D.; Proksch, P. Induced production of depsipeptides by co-culturing Fusarium tricinctum and Fusarium begoniae. Tetrahedron Lett. 2013, 54, 2492–2496. [Google Scholar] [CrossRef]
- Bentley, S.D.; Chater, K.F.; Cerdeno-Tarraga, A.M.; Challis, G.L.; Thomson, N.R.; James, K.D.; Harris, D.E.; Quail, M.A.; Kieser, H.; Harper, D.; et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3 (2). Nature 2002, 417, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Ishikawa, J.; Hanamoto, A.; Shinose, M.; Kikuchi, H.; Shiba, T.; Sakaki, Y.; Hattori, M.; Omura, S. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 2003, 21, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Ishikawa, J.; Hara, H.; Suzuki, H.; Ikenoya, M.; Ikeda, H.; Yamashita, A.; Hattori, M.; Horinouchi, S. Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J. Bacteriol. 2008, 190, 4050–4060. [Google Scholar] [CrossRef] [PubMed]
- Nett, M.; Ikeda, H.; Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liao, G.; Zhang, J.; Tan, H. Identification of novel tylosin analogues generated by a wblA disruption mutant of Streptomyces ansochromogenes. Microb. Cell Fact. 2015, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hou, L.; Li, H.; Qiu, Y.; Ju, J.; Li, W. Activation of a plasmid-situated type III PKS gene cluster by deletion of a wbl gene in deepsea-derived Streptomyces somaliensis SCSIO ZH66. Microb. Cell Fact. 2016, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, T.; Liu, H.; Fang, Y.; Gu, Q.; Zhu, W. Four butenolides are novel cytotoxic compounds isolated from the marine-derived bacterium, Streptoverticillium luteoverticillatum 11014. Arch. Pharmacal. Res. 2006, 29, 624–626. [Google Scholar] [CrossRef]
- Mukku, V.J.; Speitling, M.; Laatsch, H.; Helmke, E. New butenolides from two marine streptomycetes. J. Nat. Prod. 2000, 63, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Pauli, N.; Séquin, U.; Zähner, H. New butenolides from the photoconductivity screening of Streptomyces antibioticus (Waksman and Woodruff) Waksman and Henrici 1948. FEMS Microbiol. Lett. 1995, 126, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, Y.; Ma, K.; Li, Y.; Huang, R.; Xie, X.; Wu, S. Two new butenolides produced by an Actinomycete Streptomyces sp. Chem. Biodiver. 2014, 11, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, H.; Xu, S.; Wang, B.; Ju, J.; Tan, H.; Li, W. Activation andenhancement of Fredericamycin A production in deepsea-derived Streptomyces somaliensis SCSIO ZH66 by using ribosome engineering and response surface methodology. Microb Cell Fact. 2015, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kuang, S.; Wu, S.; Jin, W.; Sun, C. A novel polysaccharide from Sargassum integerrimum induces apoptosis in A549 cells and prevents angiogensis in vitro and in vivo. Sci. Rep. 2016, 6, 26722. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Nuzzo, G.; Hamdy, N.A.; Fakhr, I.; Banuls, L.M.; Goietsenoven, G.V.; Villani, G.; Mathieu, V.; Soest, R.V.; Kiss, R.; et al. In vitro pharmacological and toxicological effects of norterpene peroxides isolated from the Red Sea sponge Diacarnus erythraeanus on normal and cancer cells. J. Nat. Prod. 2013, 76, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
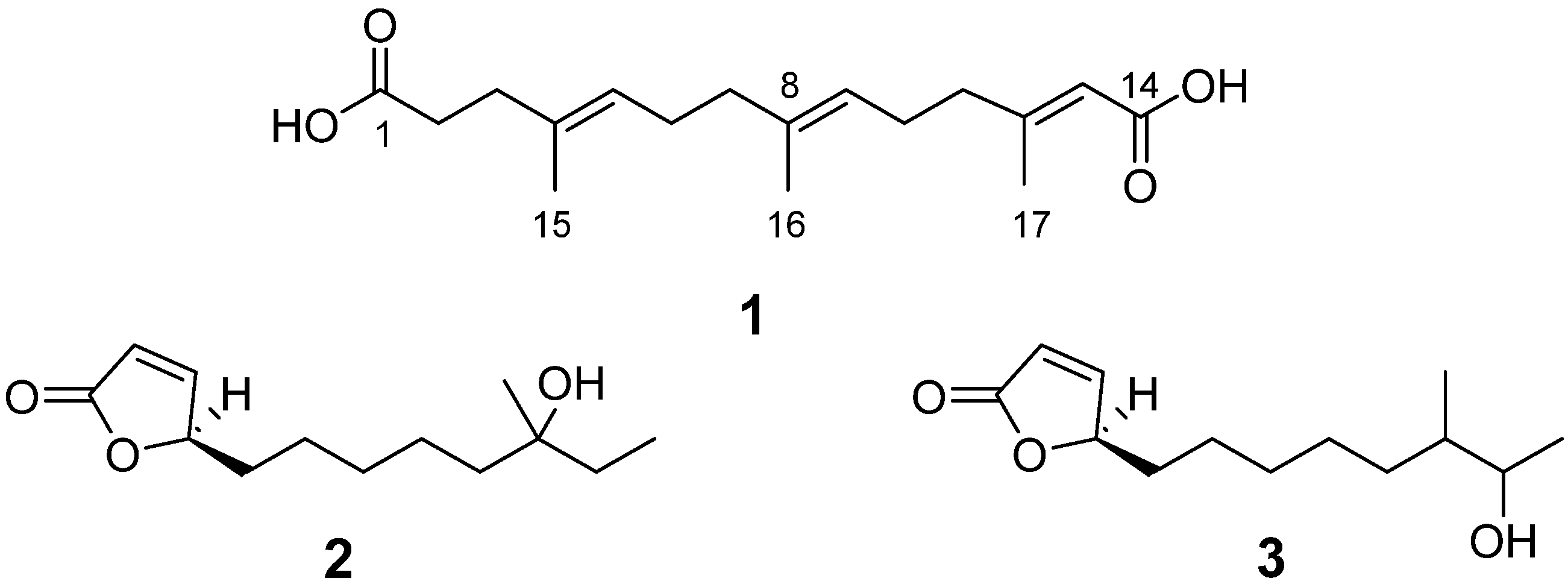
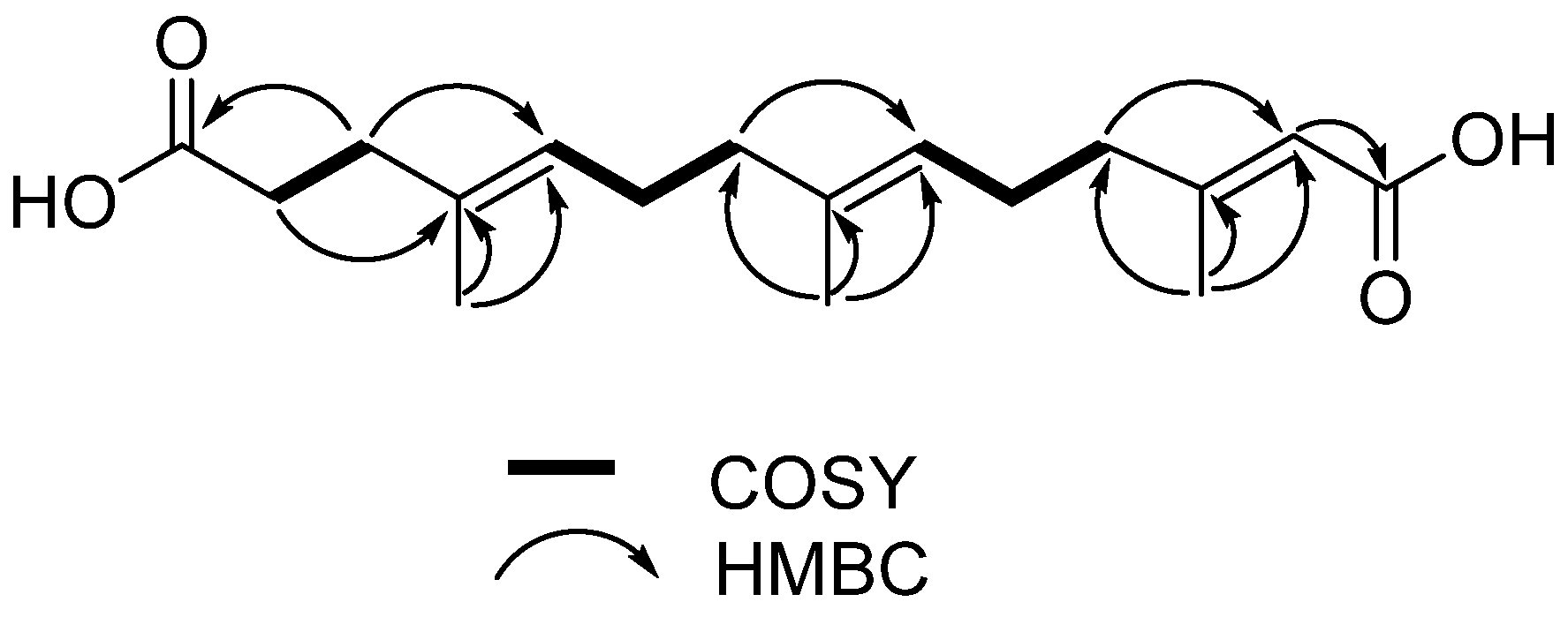
| Position | δH | δC |
|---|---|---|
| 1 | - | 174.1 |
| 2 | 2.26 (2H, m) | 32.7 |
| 3 | 2.16 (2H, t, 8.4) | 34.2 |
| 4 | - | 133.6 |
| 5 | 5.09 (1H, m) | 124.0 |
| 6 | 2.02 (2H, m) | 26.1 |
| 7 | 1.93 (2H, t, 7.8) | 39.1 |
| 8 | - | 135.2 |
| 9 | 5.07 (1H, m) | 123.1 |
| 10 | 2.12 (2H, m) | 25.5 |
| 11 | 2.12 (2H, m) | 40.0 |
| 12 | 158.4 | |
| 13 | 5.58 (1H, s) | 116.2 |
| 14 | 167.4 | |
| 15 | 1.55 (3H, s) | 15.8 |
| 16 | 1.56 (3H, s) | 15.8 |
| 17 | 2.07 (3H, s) | 18.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Li, H.; Qiu, Y.; Hou, L.; Ju, J.; Li, W. A New Dioic Acid from a wbl Gene Mutant of Deepsea-Derived Streptomyces somaliensis SCSIO ZH66. Mar. Drugs 2016, 14, 184. https://doi.org/10.3390/md14100184
Huang H, Li H, Qiu Y, Hou L, Ju J, Li W. A New Dioic Acid from a wbl Gene Mutant of Deepsea-Derived Streptomyces somaliensis SCSIO ZH66. Marine Drugs. 2016; 14(10):184. https://doi.org/10.3390/md14100184
Chicago/Turabian StyleHuang, Huiming, Huayue Li, Yanhong Qiu, Lukuan Hou, Jianhua Ju, and Wenli Li. 2016. "A New Dioic Acid from a wbl Gene Mutant of Deepsea-Derived Streptomyces somaliensis SCSIO ZH66" Marine Drugs 14, no. 10: 184. https://doi.org/10.3390/md14100184