Functional Genomics of the Aeromonas salmonicida Lipopolysaccharide O-Antigen and A-Layer from Typical and Atypical Strains
Abstract
:1. Introduction
2. Results
| Sugar Linkage | RtGM a (min) | Relative Molar Ratios b |
|---|---|---|
| 4-Substituted Rha | 5.19 | 0.07 |
| Terminal Glc | 6.18 | 1.00 |
| 3,4-Substituted Rha | 7.03 | 0.85 |
| 3-Substituted ManNAc | 34.81 | 0.60 |

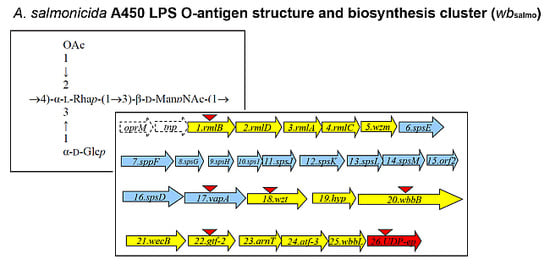
2.1. LPS O-Antigen Biosynthesis Gene Cluster (wbsalmo)
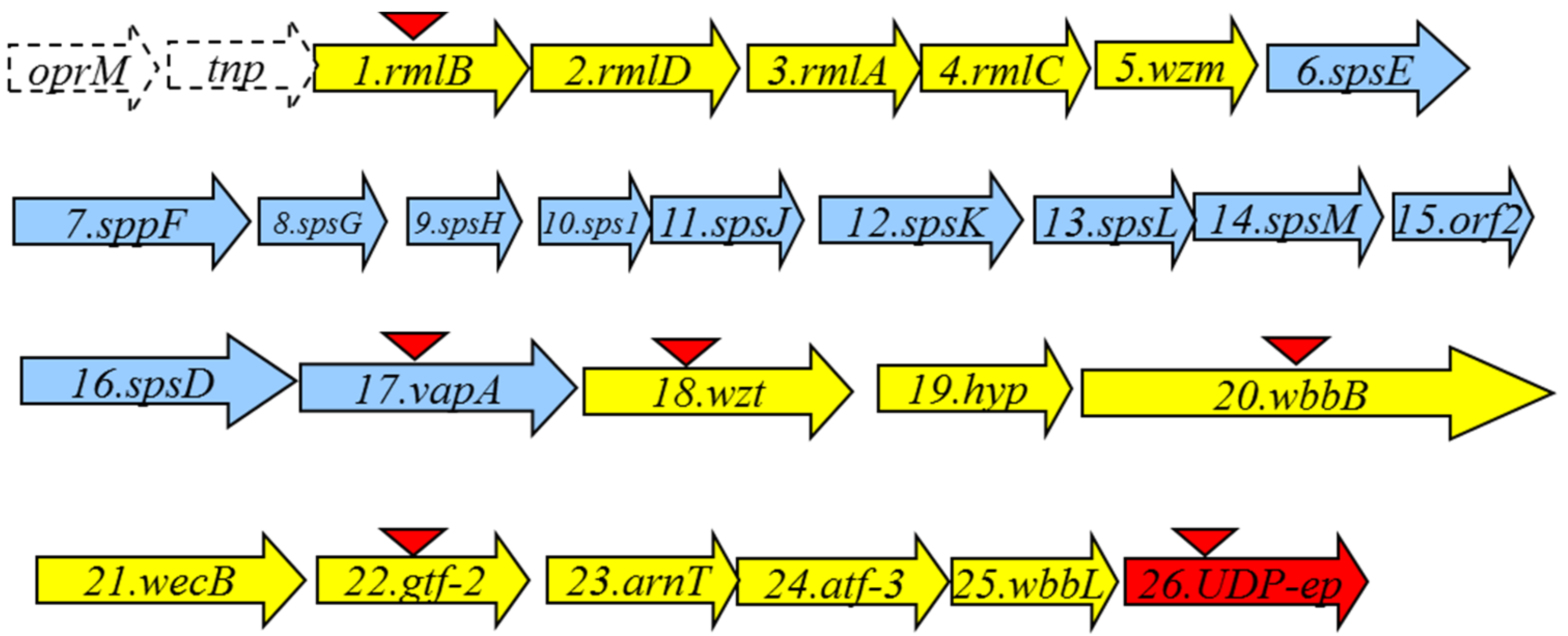
| ORF | Protein Name | Protein Size (in Amino Acid Residues) | Predicted Function | Homologous Protein with Known Function | Percentage in Amino Acid Identity/Similarity |
|---|---|---|---|---|---|
| Inserted S-layer protein cluster | |||||
| 18 | Wzt | 438 | ABC transporter ATP binding protein | Wzt multispecies Aeromonas | 100/100 |
| 19 | Hyp | 216 | Hypothetical protein with domain Sulfotransferase | Sulfotransferase Vibrio cholerae | 38/54 |
| 20 | WbbB | 1122 | N-acetyl glucosaminyl transferase | WbbB Klebsiella pneumonaie | 63/77 |
| 21 | WecB | 370 | UDP-N-acetyl glucosamine 2-epimerase | WecB Serratia marcescens | 100/100 |
| 22 | Gtf-2 | 355 | Glycosyl transferase | Glycosyl transferase family group 2 Vibrio choleare | 78/91 |
| 23 | ArnT | 457 | Hypothetical protein with ArnT (4-amino-4-deoxy-l-arabinose transferase) domain | Hypothetical protein multispecies Aeromonas | 100/100 |
| 24 | Atf-3 | 348 | Acetyl transferase family 3 | Acetyltransferase Serratia marcescens | 45/65 |
| 25 | WbbL | 288 | Rhamnosyl transferase | -Glucosyl transferase family 2 A. veronii -Rhamnosyl transferase E. coli | -100/100 -43/67 |
| 26 | UDP-ep | 318 | NAD-dependent dehydratase or UDP-sugar epimerase | NAD-dependent dehydratase or UDP-sugar epimerase multispecies Aeromonas | 100/100 |
| A-layer protein cluster | |||||
| 6 | SpsE | 552 | S-layer secretion system protein E | Type II secretion system (T2SS) protein E A. salmonicida | 100/100 |
| 7 | SpsF | 395 | S-layer secretion system protein F | Type II secretion system(T2SS) protein F A. salmonicida | 100/100 |
| 8 | SpsG | 143 | S-layer secretion system protein G | Type II secretion system (T2SS) protein G A. salmonicida | 97/99 |
| 9 | SpsH | 131 | S-layer secretion system protein H | Type II secretion system (T2SS) protein H A. salmonicida | 96/98 |
| 10 | SpsI | 132 | S-layer secretion system protein I | Type II secretion system (T2SS )protein I A. salmonicida | 99/100 |
| 11 | SpsJ | 235 | S-layer secretion system protein J | Type II secretion system (T2SS) protein J A. salmonicida | 94/98 |
| 12 | SpsK | 288 | S-layer secretion system protein K | Type II secretion system (T2SS) protein K A. salmonicida | 100/100 |
| 13 | SpsL | 371 | S-layer secretion system protein L | Type II secretion system (T2SS) protein L A. salmonicida | 94/95 |
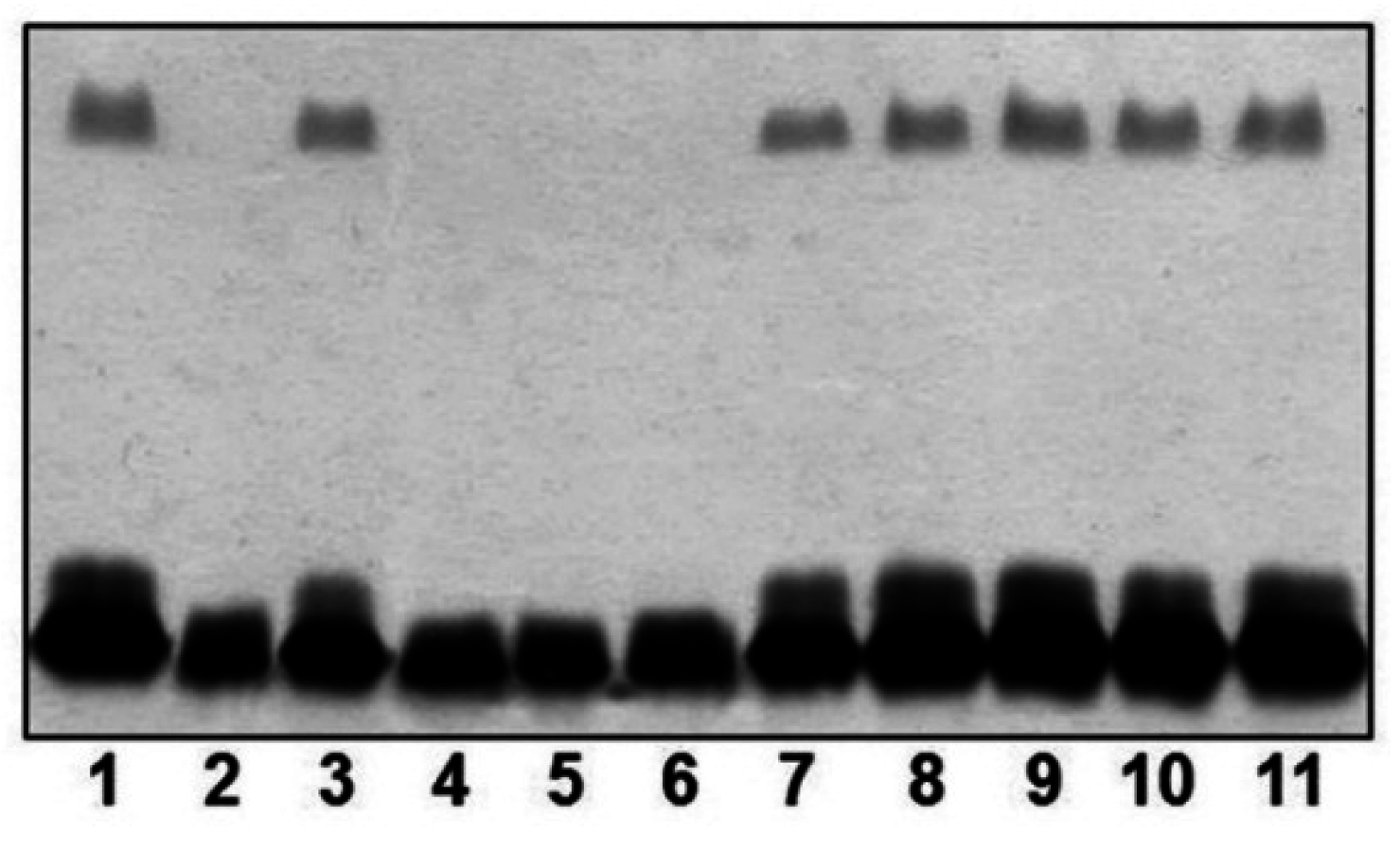
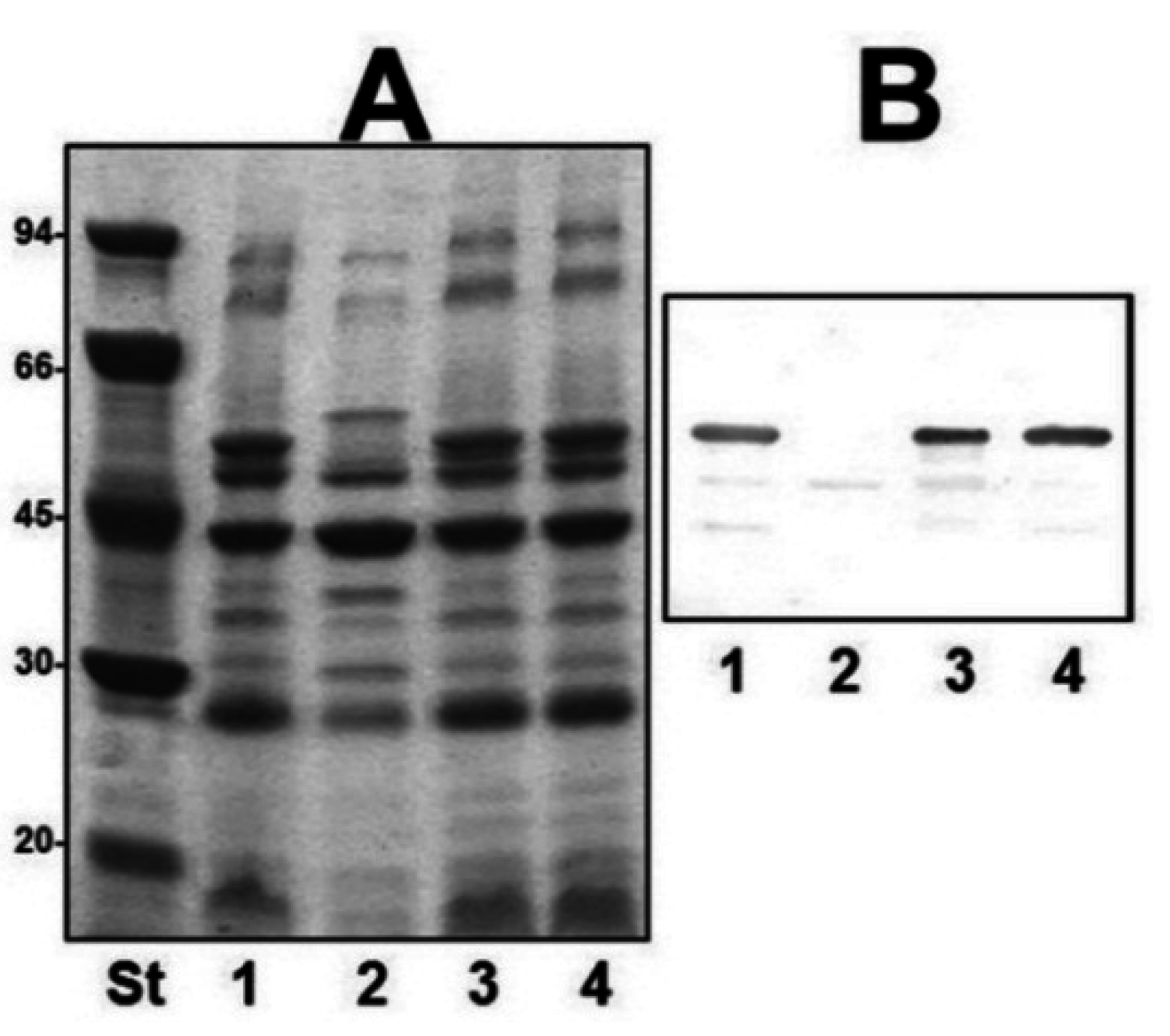
2.2. Mutant Isolation and Characterization
2.3. Different A. salmonicida Subspecies Strains
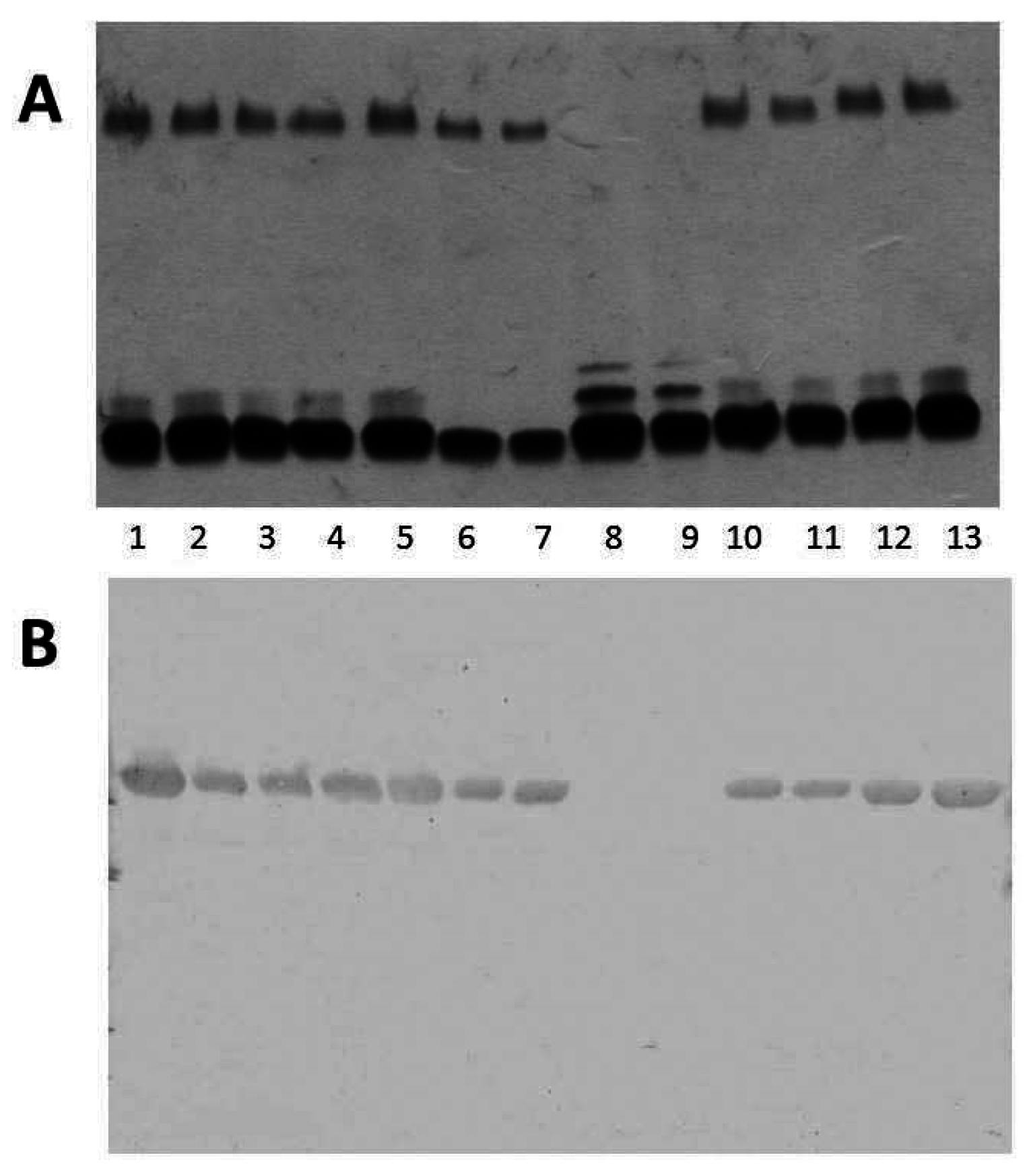
2.4. Analysis of Fully Sequenced Genomes
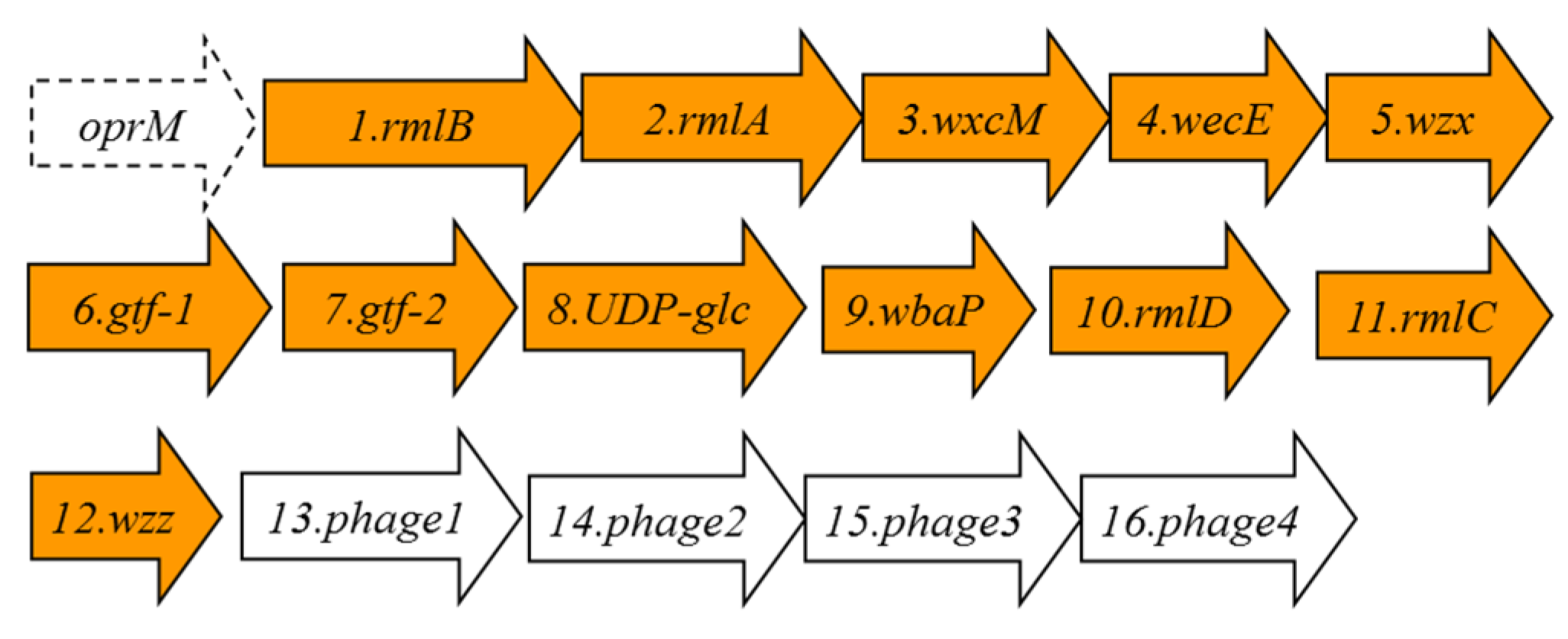
| ORF | Protein Name | Protein Size in Amino Acid Residues | Predicted Function |
|---|---|---|---|
| 1 | RmlB | 361 | dTDP-glucose-4-6-dehydratase RmlB |
| 2 | RmlA | 289 | Glucose-1-phosphate thymidylyl transferase RmlA |
| 3 | WxcM | 137 | dTDP-6-deoxy-3,4-keto-hexulose isomerase. |
| 4 | WecE | 367 | aminotransferase family, WecE |
| 5 | Wzx | 416 | O-antigen flippase |
| 6 | Gtf-1 | 140 | glycosyl transferase group 1 |
| 7 | Gtf-2 | 249 | glycosyl transferase group 2 |
| 8 | UDP-glc | 388 | UDP-glucose 6-dehydrogenase |
| 9 | WbaP | 423 | polyprenyl glycosyl phosphotransferase |
| 10 | RmlD | 296 | dTDP-4-dehydro rhamnose reductase |
| 11 | RmlC | 176 | dTDP-4-dehydro rhamnose 3,5-epimerase |
| 12 | Wzz | 202 | O-antigen size regulator protein |
| 13 | Phage1 | 113 | Phage terminase 1 protein |
| 14 | Phage2 | 283 | phage portal protein |
| 15 | Phage3 | 141 | phage prohead peptidase |
| 16 | Phage4 | 398 | putative phage phi-C31 gp36 major capsid-like protein |
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids and Growth Conditions
4.1.1. General DNA Methods
4.1.2. DNA Sequencing and Computer Analysis of Sequence Data
| Strain or Plasmid | Relevant Characteristics | Reference or Source |
|---|---|---|
| E. coli strains | ||
| DH5α | F− end A hsdR17 (rK− mK+) supE44 thi-1 recA1 gyr-A96 _80lacZM15 | [25] |
| MC1061 | thi thr1 leu6 proA2 his4 argE2 lacY1 galK2 ara14 xyl5, supE44, λpir | [26] |
| A. salmonicida strains | ||
| A450 | Wild type, subsp. salmonicida | [27] |
| A450nal R | A450 nalidixic acid resistant | [27] |
| A450ΔrmlB | A450 rmlB in frame mutant unable to produce LPS O-antigen | This study |
| A450Δwzt | A450 wzt in frame mutant unable to produce LPS O-antigen | This study |
| A450ΔwbbB | A450 wbbB in frame mutant unable to produce LPS O-antigen | This study |
| A450Δgtf-2 | A450 gtf-2 in frame mutant unable to produce LPS O-antigen | This study |
| A450ΔvapA | A450 vapA in frame mutant, unable to produce A-layer but able to produce LPS O-antigen | This study |
| A450ΔUDP-ep | A450 UDP-ep in frame mutant, able to produce LPS O-antigen and A-layer | This study |
| CECT894 | Wild type, subsp. salmonicida | CECT |
| CECT4235 | Wild type, subsp. salmonicida | CECT |
| CECT896T | Wild type, subsp. masoucida | CECT |
| AS60 | Wild type, subsp. masoucida | [28] |
| CECT4238 | Wild type, subsp. achromogenes | CECT |
| CECT895T | Wild type, subsp. achromogenes | CECT |
| AS46 | Wild type, subsp. achromogenes | [28] |
| AS102 | Wild type, subsp. achromogenes | [28] |
| CECT5752T | Wild type, subsp. pectinolytica | CECT |
| CECT5753 | Wild type, subsp. pectinolytica | CECT |
| CECT5179 | Wild type, subsp. smithia | CECT |
| AS74 | Wild type, subsp. smithia | [28] |
| Plasmids | ||
| pGEMT easy | PCR generated DNA fragment cloning vector Amp R | Promega |
| pBAD33-Gm | Arabinose-inducible expression vector, Gm R | [27,29] |
| pDM4 | pir dependent with sacAB genes; oriR6K; Cm R | [27] |
| pLA2917 | Cosmid vector, Km R, Tc R | [15] |
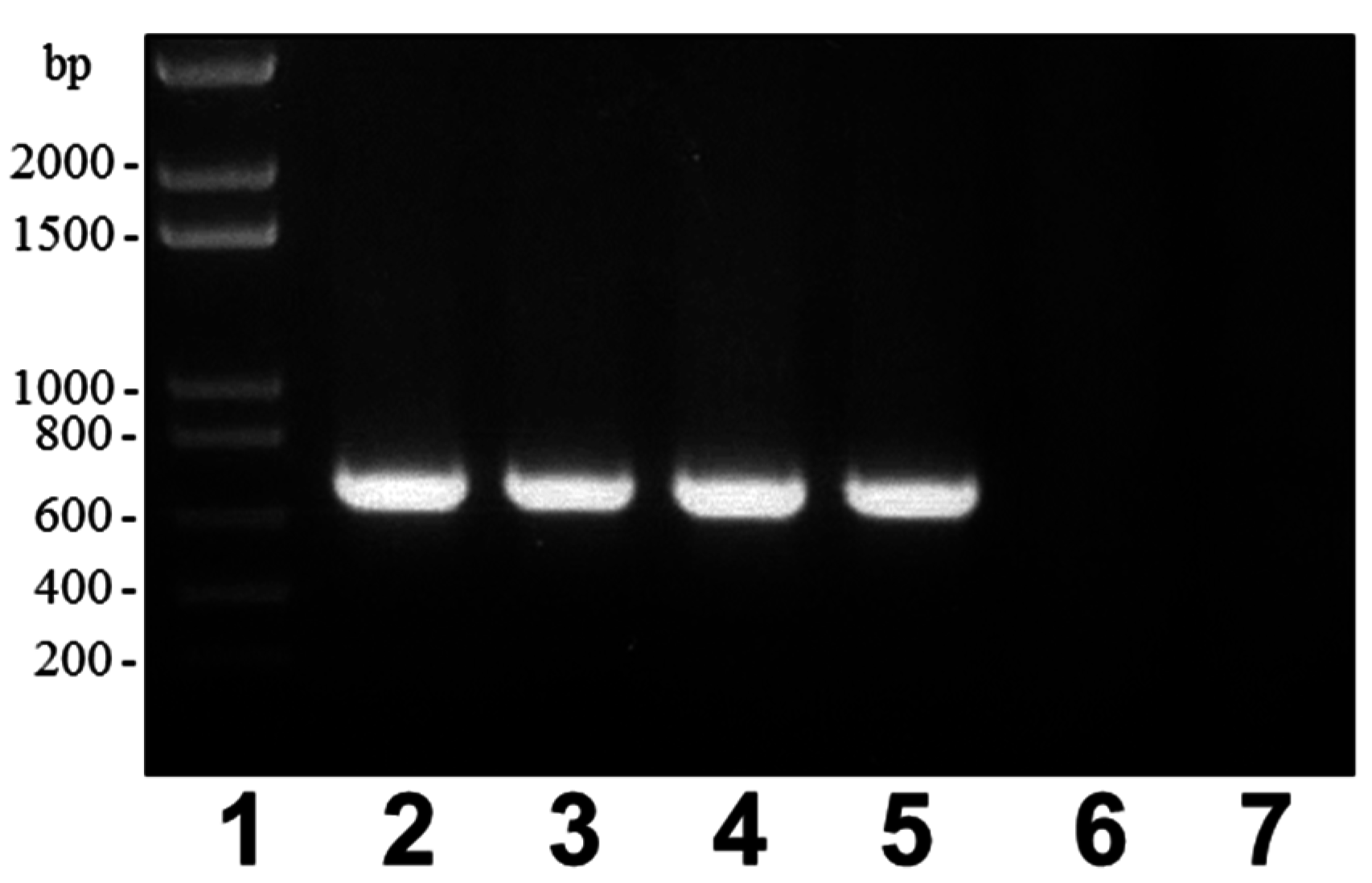
4.1.3. Mutant and Plasmid Constructions, Mutant Complementation Studies
| A | |
|---|---|
| Primers a,b | Amplified Fragment |
| rmlB | |
| A: 5′-CGCGGATCCCAAGTTCTGCCTGGTAT-3′ | AB (632 bp) |
| B: 5′-TGTTTAAGTTTAGTGGATGGGTGCACCACCAGTGACAAG-3′ | |
| C: 5′-CCCATCCACTAAACTTAAACAAGTGGTGCCTACCAATCCT-3′ | CD (704 bp) |
| D: 5′-CGCGGATCCAACATCGGGTTTGCTCT-3′ | |
| AD (1312 bp) | |
| vapA | |
| A: 5′-GAAGATCTGCCGATTCAGGTAAAACAG-3′ | AB (717 bp) |
| B: 5′-TGTTTAAGTTTAGTGGATGGGGCTAATCACGACATCAGCA-3′ | |
| C: 5′-CCCATCCACTAAACTTAAACA GAAGGCGTGGATATTCAGA-3′ | CD (670 bp) |
| D: 5′-GAAGATCTAACGATCATCCATCTCTCG-3′ | |
| AD (1366 bp) | |
| wzt | |
| A: 5′-CGCGGATCCGAGCTGGCTGATCTCTTCA-3′ | AB (721 bp) |
| B: 5′-TGTTTAAGTTTAGTGGATGGGGGAACGATAGATGGGAAATG-3′ | |
| C: 5′-CCCATCCACTAAACTTAAACAGATGTCGCCATGTTTCAAG-3′ | CD (653 bp) |
| D: 5′-CGCGGATCCTGATTGGGCGAAAATA-3′ | |
| AD (1353 bp) | |
| wbbB | |
| A: 5′-CGCGGATCCTACTTGCCCGAGATACCAG-3′ | AB (659 bp) |
| B: 5′-TGTTTAAGTTTAGTGGATGGGACCTAGCACGACCCAAAG-3′ | |
| C: 5′-CCCATCCACTAAACTTAAACAGTTAAGCAGGCGCTATTTG-3′ | CD (753 bp) |
| D: 5′-CGCGGATCCTACGATGCGATGTTACCAA-3′ | |
| AD (1391 bp) | |
| gtf-2 | |
| A: 5′-CGCGGATCCGCACCTACGCAAATTTCTC-3′ | AB (722 bp) |
| B: 5′-TGTTTAAGTTTAGTGGATGGGCACCGGTGAAAGATAAACC-3′ | |
| C: 5′-CCCATCCACTAAACTTAAACATTTCATAATAGTGGCGATGC-3′ | CD (631bp) |
| D: 5′-CGCGGATCCGACTGCCGTCTCTTTGAAC-3′ | |
| AD (1332 bp) | |
| UDP-ep | |
| A: 5′-CGCGGATCCTGGCGTTGAATAATGGAG-3′ | AB (646 bp) |
| B: 5′-TGTTTAAGTTTAGTGGATGGGCTTACCAACAAACCCGTTG-3′ | |
| C: 5′-CCCATCCACTAAACTTAAACAAAGGCTCAGAGGCGATTAC-3′ | CD (771 bp) |
| D: 5′-CGCGGATCCACCATCCCCCATAAAGAT-3′ | |
| AD (1395 bp) | |
| B | ||
|---|---|---|
| Plasmid | Primers | Amplified Fragment |
| pBADGm-rmlB c | RmlB-FOR: 5′-TCCCCCGGGTTAAAAGCAGCGAACTG-3′ | 1380 bp |
| RmlB-REV: 5′-GCTCTAGACGCTGGAGTCAAAATCAAC-3′ | ||
| pBADGm-vapA c | VapA-FOR: 5′-TCCCCCGGGTGATCAACGGATAGGTTCAA-3′ | 1666 bp |
| VapA-REV: 5′-GCTCTAGAAGGGAACAAATGAAACTGCT-3′ | ||
| pBADGm-wzt c | Wzt-FOR: 5′-TCCCCCGGGTGACCACAGCCCTTATTTC-3′ | 1473 bp |
| Wzt-REV: 5′-GCTCTAGATGCAGTAGTCCCACCTTTT-3′ | ||
| pBADGm-wbbB d | WbbB-FOR: 5′GGAATTCTAAGCTCACGGTTGCACAG-3′ | 3689 bp |
| WbbB-REV: 5′-TCCCCCGGGATAACCGGAGCCATTTTGAT-3′ | ||
| pBADGm-gtf2 c | gtf2-FOR: 5′-TCCCCCGGGATGGCTAAAGGTTCTTCACC-3′ | 1269 bp |
| gtf2-REV: 5′-GCTCTAGACATGACTGAAATACCCTGGA-3′ | ||
4.1.4. LPS Characterization and SDS-PAGE
4.1.5. LPS Isolation and O-Deacetylation
4.1.6. OM Protein and S-Layer Isolation and Characterization
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Austin, B.; Austin, D.A. Characteristics of the pathogens: Gram-negative bacteria. In Bacterial Fish Pathogens: Diseases of Farmed and Wild Fish; Austin, B., Austin, D.A., Eds.; Springer Praxis Publishing: Chichester, UK, 2007; pp. 81–150. [Google Scholar]
- Scott, M. The pathogenicity of Aeromonas salmonicida (Griffin) in sea and brackish waters. J. Gen. Microbiol. 1968, 50, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, E.E.; Kay, W.W.; Ainsworth, T.; Chamberlain, J.B.; Buckley, J.T.; Trust, T.J. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J. Bacteriol. 1981, 148, 393–400. [Google Scholar]
- Belland, R.J.; Trust, T.J. Synthesis, export, and assembly of Aeromonas salmonicida A-layer analysed by transposon mutagenesis. J. Bacteriol. 1985, 163, 877–881. [Google Scholar] [PubMed]
- Dooley, J.S.G.; Engelhardt, H.; Baumeister, W.; Kay, W.W.; Trust, T.J. Three dimensional structure of an open form of the surface layer from the fish pathogen Aeromonas salmonicida. J. Bacteriol. 1989, 171, 190–197. [Google Scholar] [PubMed]
- Phipps, B.M.; Kay, W.W. Immunoglobulin binding by the regular surface array of Aeromonas salmonicida. J. Biol. Chem. 1988, 263, 9298–9303. [Google Scholar] [PubMed]
- Munn, C.B.; Ishiguro, E.E.; Kay, W.W.; Trust, T.J. Role of the surface components in serum resistance of virulent Aeromonas salmonicida. Infect. Immun. 1982, 36, 1069–1075. [Google Scholar] [PubMed]
- Merino, S.; Albertí, S.; Tomás, J.M. Aeromonas salmonicida resistance to complement-mediated killing. Infect. Immun. 1994, 62, 5483–5490. [Google Scholar] [PubMed]
- Aquilini, E.; Tomás, J.M. Lipopolysaccharides (Endotoxins). In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2015; in press. [Google Scholar]
- Shaw, D.H.; Lee, Y.Z.; Squires, M.J.; Lüderitz, O. Structural studies on the O-antigen of Aeromonas salmonicida. Eur. J. Biochem. 1983, 131, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.J.; Lüderitz, O. Structure of the core oligosaccharide in the lipopolysaccharide isolated from Aeromonas salmonicida ssp. salmonicida. Carbohydr. Res. 1992, 231, 83–91. [Google Scholar]
- Wang, Z.; Vinogradov, E.; Larocque, S.; Harrison, B.A.; Li, J.; Altman, E. Structural and serological characterization of the O-chain polysaccharide of Aeromonas salmonicida strains A449, 80204 and 80204-1. Carbohydr. Res. 2005, 340, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Vinogradov, E.; Altman, E. Structural studies of the core region of Aeromonas salmonicida subsp. salmonicida lipopolysaccharide. Carbohydr. Res. 2006, 341, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Westphal, O.; Jann, K. Bacterial lipopolysaccharide extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 1965, 5, 83–89. [Google Scholar]
- Nogueras, M.M.; Merino, S.; Aguilar, A.; Benedí, V.J.; Tomás, J.M. Cloning, sequencing and role in serum susceptibility of porin II from mesophilic Aeromonas sp. Infect. Immun. 2000, 68, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, N.; Canals, R.; Saló, M.T.; Vilches, S.; Merino, S.; Tomás, J.M. The Aeromonas hydrophila wb*O34 gene cluster: Genetics and temperature regulation. J. Bacteriol. 2008, 190, 4198–4209. [Google Scholar] [CrossRef] [PubMed]
- Artsimovitch, I.; Landick, R. The transcriptional regulator RfaH stimulates RNA chain synthesis after recruitment to elongation complexes by the exposed nontemplate DNA strand. Cell 2002, 109, 193–203. [Google Scholar] [CrossRef]
- Bailey, M.J.; Hughes, C.; Koronakis, V. RfaH and the ops element, components of a novel system controlling bacterial transcription elongation. Mol. Microbiol. 1997, 26, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Morales, F.; Prieto, A.I.; Beuzón, C.R.; Holden, D.W.; Casadesús, J. Role for Salmonella enterica Enterobacterial Common Antigen in Bile Resistance and Virulence. J. Bacteriol. 2003, 185, 5328–5332. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.; Kanehisa, M.; DeLisi, C. The detection and classification of membrane-spanning proteins. Biochim. Biophys. Acta 1985, 815, 468–476. [Google Scholar] [CrossRef]
- Izquierdo, L.; Merino, S.; Regué, M.; Rodríguez, F.; Tomás, J.M. A Klebsiella pneumoniae O-antigen heteropolysaccharide (O12) requiring an ABC-2-trasnporter dependent pathway. J. Bacteriol. 2003, 185, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Saigi, F.; Climent, N.; Piqué, N.; Sanchez, C.; Merino, S.; Rubirés, X.; Aguilar, A.; Tomás, J.M.; Regué, M. Genetic analysis of the Serratia marcescens N28b O4 antigen gene cluster. J. Bacteriol. 1999, 181, 1883–1891. [Google Scholar] [PubMed]
- Knirel, Y.A.; Vinogradov, E.; Jimenez, N.; Merino, S.; Tomás, J.M. Structural studies on the R-type lipopolysaccharide of Aeromonas hydrophila. Carbohydr. Res. 2004, 339, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, B.K. Infections by atypical strains of the bacterium Aeromonas salmonicida. Iceland Agric. Sci. 1998, 12, 61–72. [Google Scholar]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Milton, D.L.; O’Toole, R.; Horstedt, P.; Wolf-Watz, H. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 1996, 178, 1310–1319. [Google Scholar]
- Jiménez, N.; Lacasta, A.; Vilches, S.; Reyes, M.; Vazquez, J.; Aquillini, E.; Merino, S.; Regué, M.; Tomás, J.M. Genetics and proteomics of Aeromonas salmonicida Lipopolysaccharide Core Biosynthesis. J. Bacteriol. 2009, 191, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.; Austin, D.A.; Dalsgaard, I.; Gudmundsdottir, B.K.; Høie, S.; Thornton, J.M.; Larsen, J.L.; O’Hici, B.; Powell, R. Characterization of atypical Aeromonas salmonicida by different methods. Syst. Appl. Microbiol. 1998, 21, 50–64. [Google Scholar] [CrossRef]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, P.J.; Brown, T.M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 1983, 154, 269–277. [Google Scholar] [PubMed]
- Sawardeker, J.S.; Sloneker, J.H.; Jeanes, A. Spectrophotometric determination of high molecular weight quaternary ammonium cations in polysaccharides. Anal. Chem. 1965, 37, 243–246. [Google Scholar]
- Widmalm, G.; Leontein, K. Structural studies of the Escherichia coli O127 O-antigen polysaccharide. Carbohydr. Res. 1993, 247, 87–89. [Google Scholar] [CrossRef]
- Esteve, C.; Amaro, C.; Toranzo, A.E. O-serogrouping and surface components of Aeromonas hydrophila and Aeromonas jandaei pathogenic for eels. FEMS Microbiol. Lett. 1994, 117, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Aguilar, A.; Rubires, X.; Simón-Pujol, D.; Congregado, F.; Tomás, J.M. The role of the capsular polysaccharide of Aeromonas salmonicida in the adherence and invasion of fish cell lines. FEMS Microbiol. Lett. 1996, 142, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Vilches, S.; Jiménez, N.; Merino, S.; Tomás, J.M. The Aeromonas dsbA mutation decreased their virulence by triggering type III secretion system but not flagella production. Microb. Pathog. 2012, 52, 130–139. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino, S.; De Mendoza, E.; Canals, R.; Tomás, J.M. Functional Genomics of the Aeromonas salmonicida Lipopolysaccharide O-Antigen and A-Layer from Typical and Atypical Strains. Mar. Drugs 2015, 13, 3791-3808. https://doi.org/10.3390/md13063791
Merino S, De Mendoza E, Canals R, Tomás JM. Functional Genomics of the Aeromonas salmonicida Lipopolysaccharide O-Antigen and A-Layer from Typical and Atypical Strains. Marine Drugs. 2015; 13(6):3791-3808. https://doi.org/10.3390/md13063791
Chicago/Turabian StyleMerino, Susana, Elena De Mendoza, Rocío Canals, and Juan M. Tomás. 2015. "Functional Genomics of the Aeromonas salmonicida Lipopolysaccharide O-Antigen and A-Layer from Typical and Atypical Strains" Marine Drugs 13, no. 6: 3791-3808. https://doi.org/10.3390/md13063791