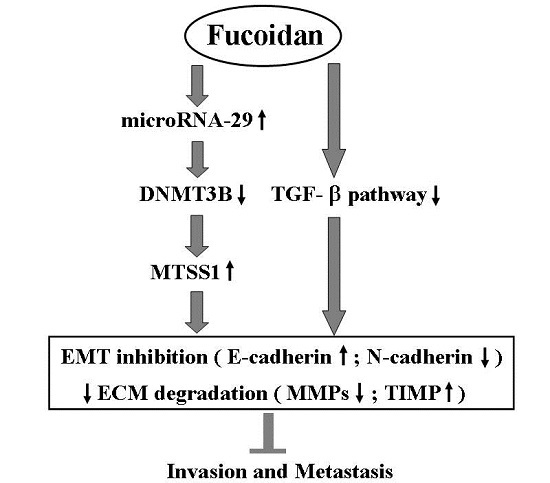
Fucoidan Elevates MicroRNA-29b to Regulate DNMT3B-MTSS1 Axis and Inhibit EMT in Human Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Results
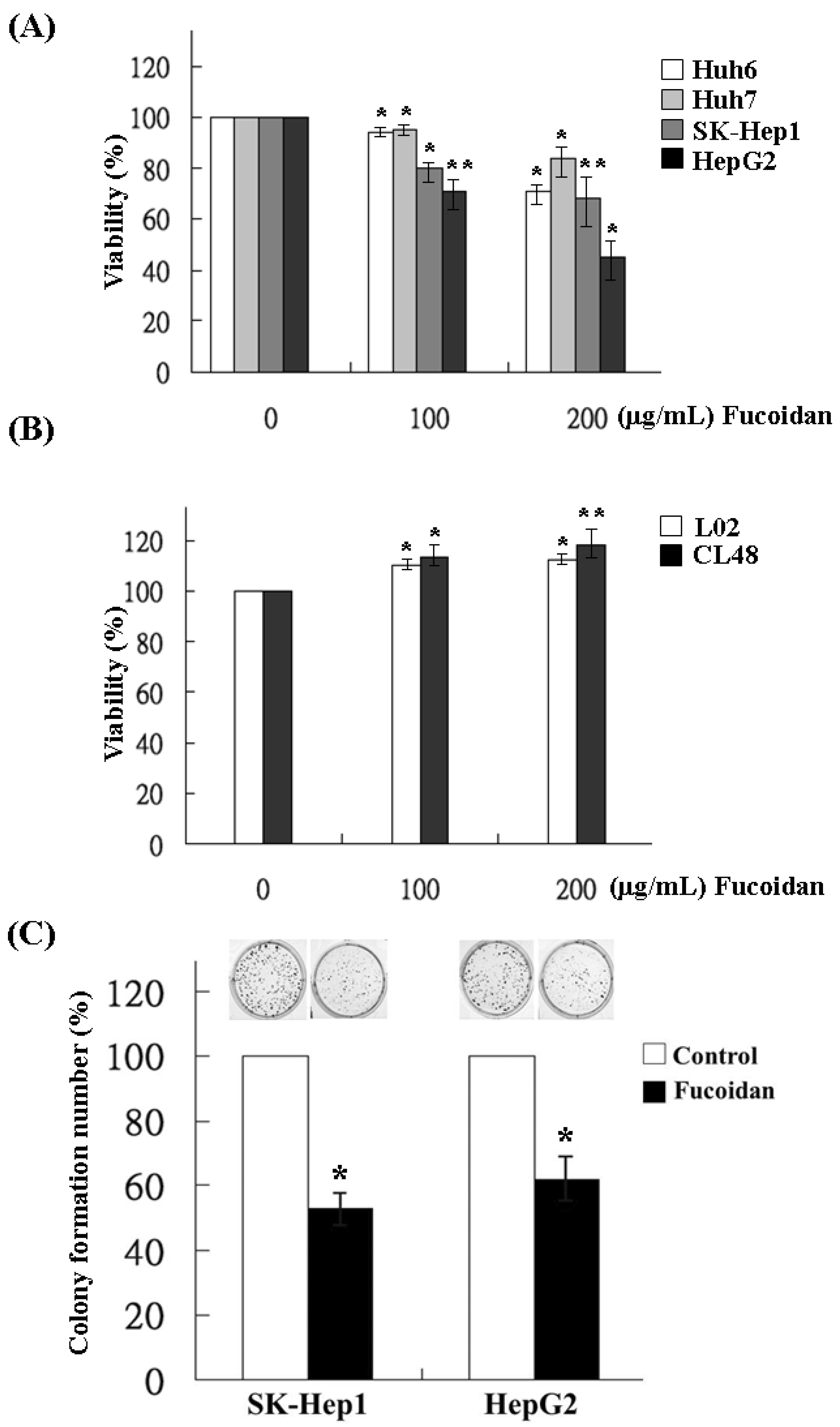
2.1. Effects of Fucoidan on the Growth and Clonogenicity of Human HCC Cells
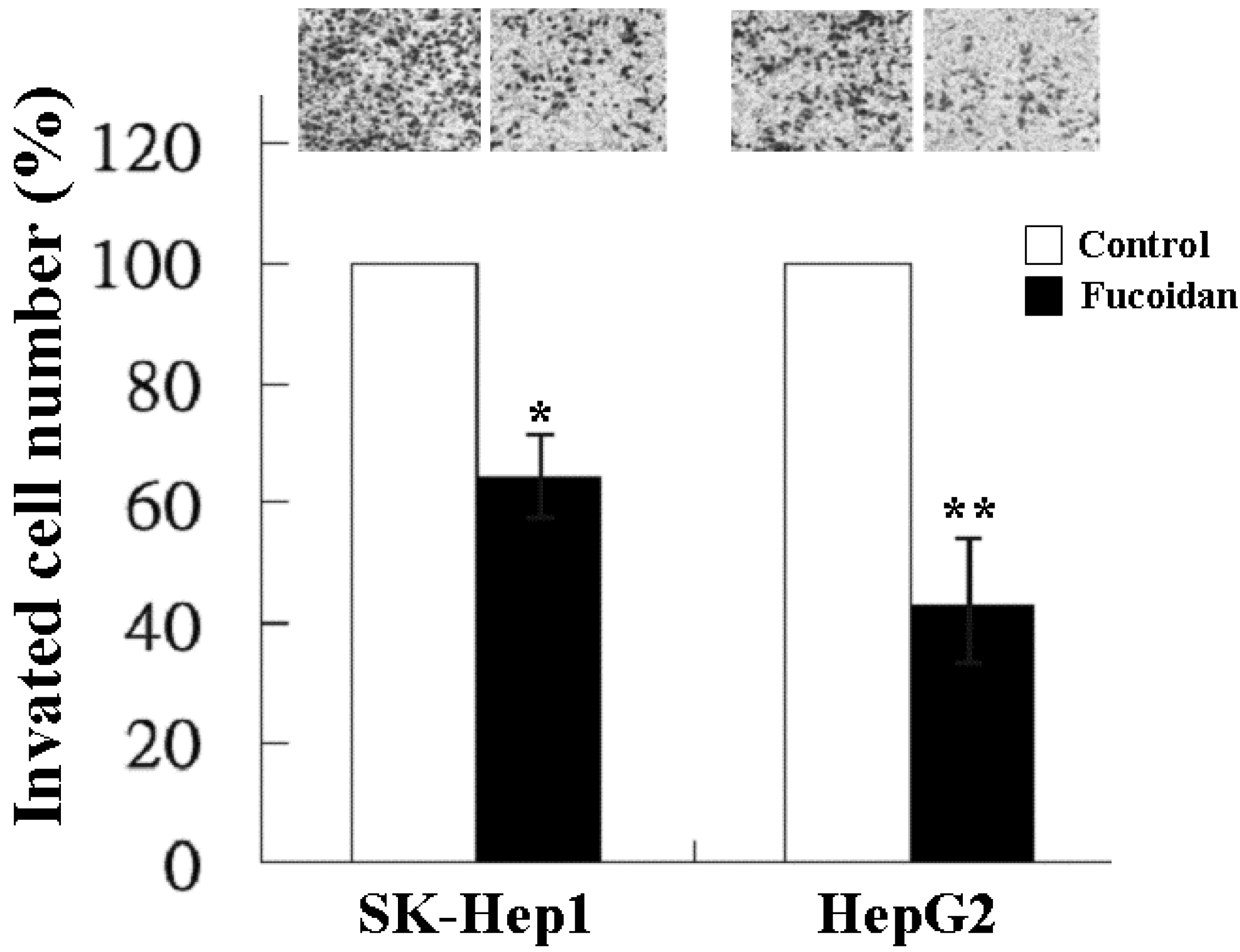
2.2. Inhibitory Effect of Fucoidan on Cell Invasion
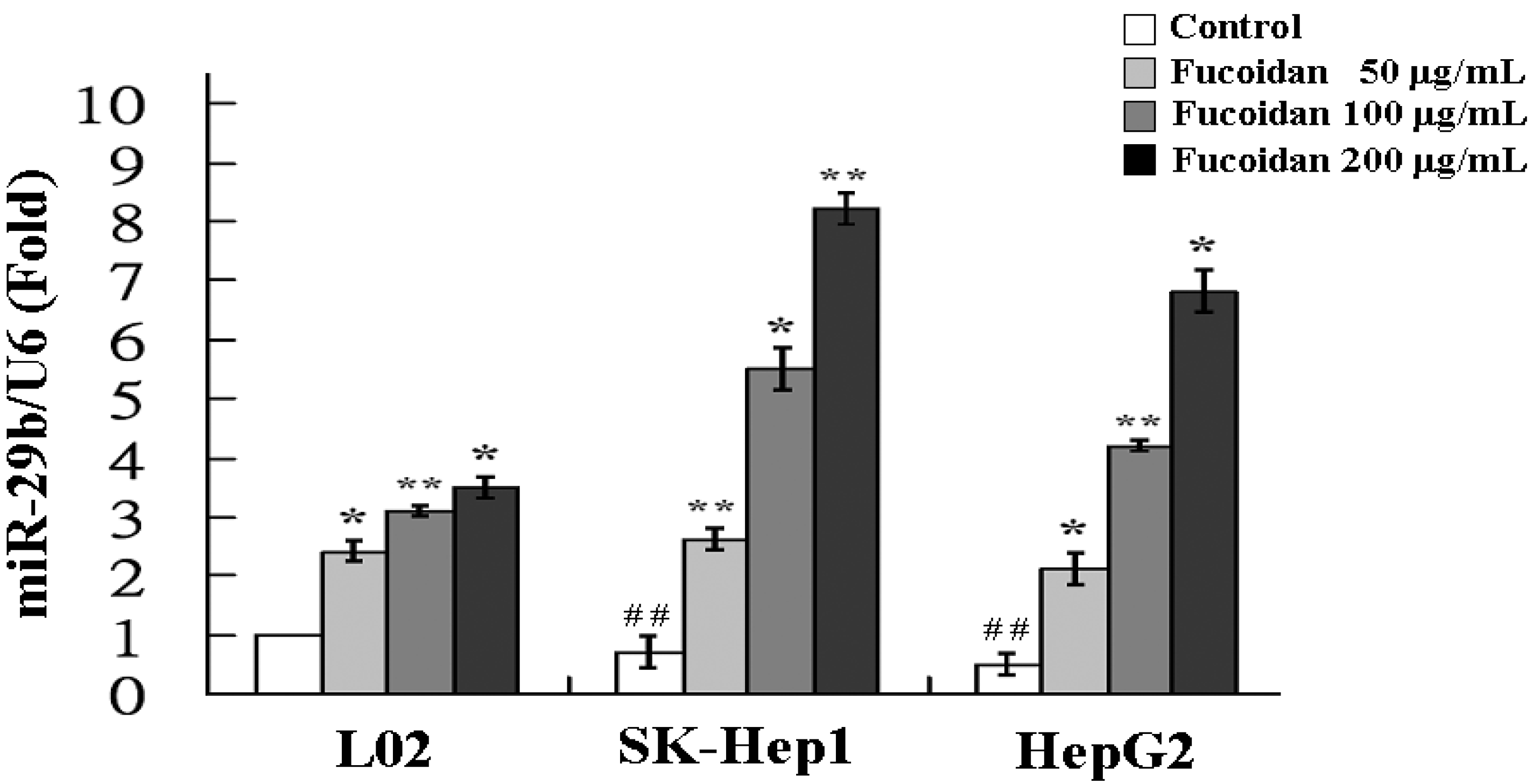
2.3. Fucoidan Increases the Expression of MiR-29b
| miR-ID | Increased Fold-Changes | miR-ID | Decreased Fold-Changes |
|---|---|---|---|
| miR-29b | 8.5 | miR-17 | 15.7 |
| miR-29a | 7.7 | miR-92a | 13.6 |
| miR-29c | 7.3 | miR-18a | 12.0 |
| miR-1224 | 6.6 | miR-192 | 7.2 |
| miR-133b | 2.0 | miR-127 | 2.4 |
| miR-200c | 1.7 | miR-154 | 1.9 |
| miR-200a | 1.4 | miR-21 | 1.7 |
| miR-205 | 1.3 | miR-680 | 1.3 |
| miR-208a | 1.2 | miR-377 | 1.2 |
| miR-669b | 1.2 | miR-153 | 1.1 |
2.4. Fucoidan Increases MiR-29b Expression to Suppress the DNMT3B
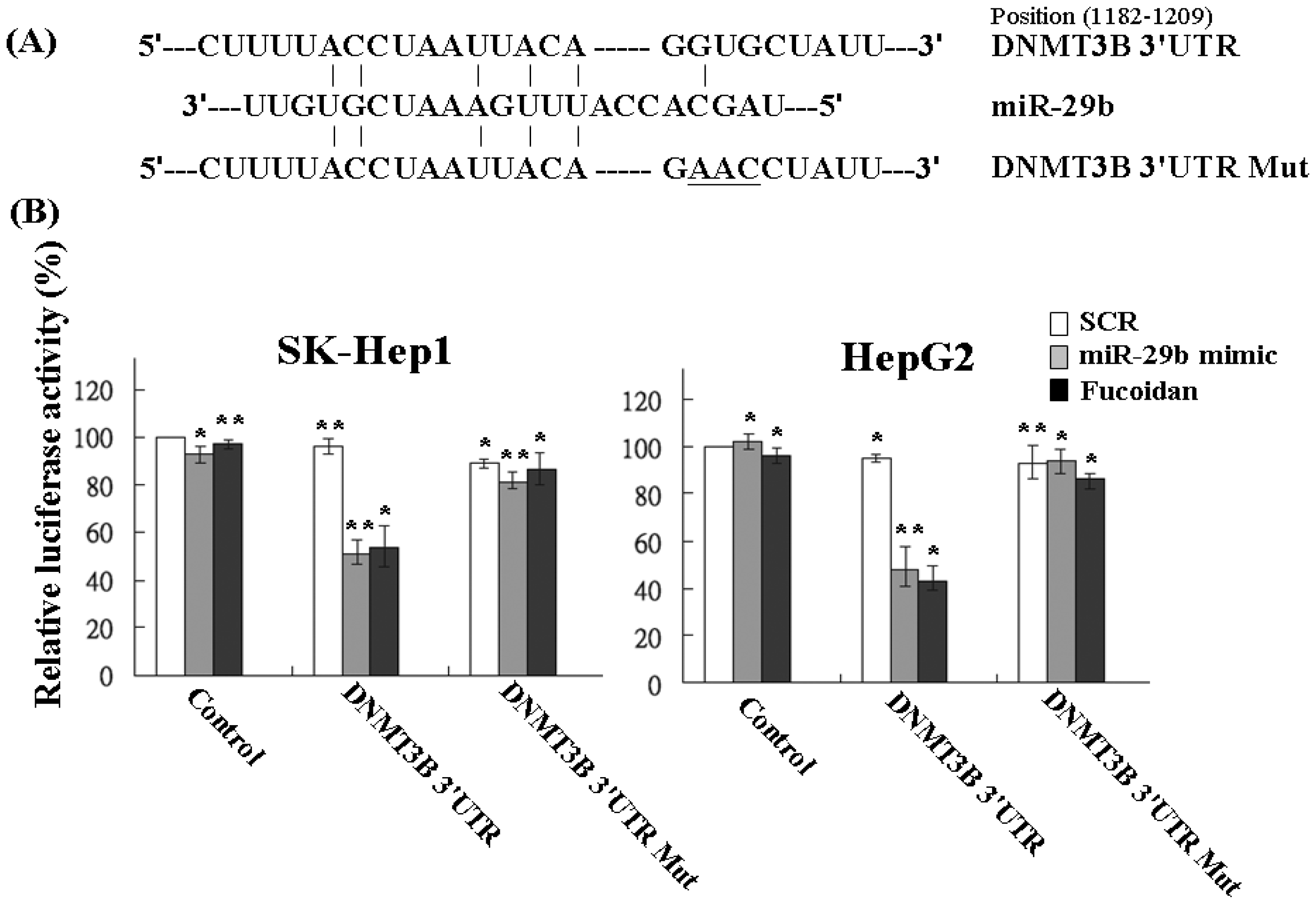
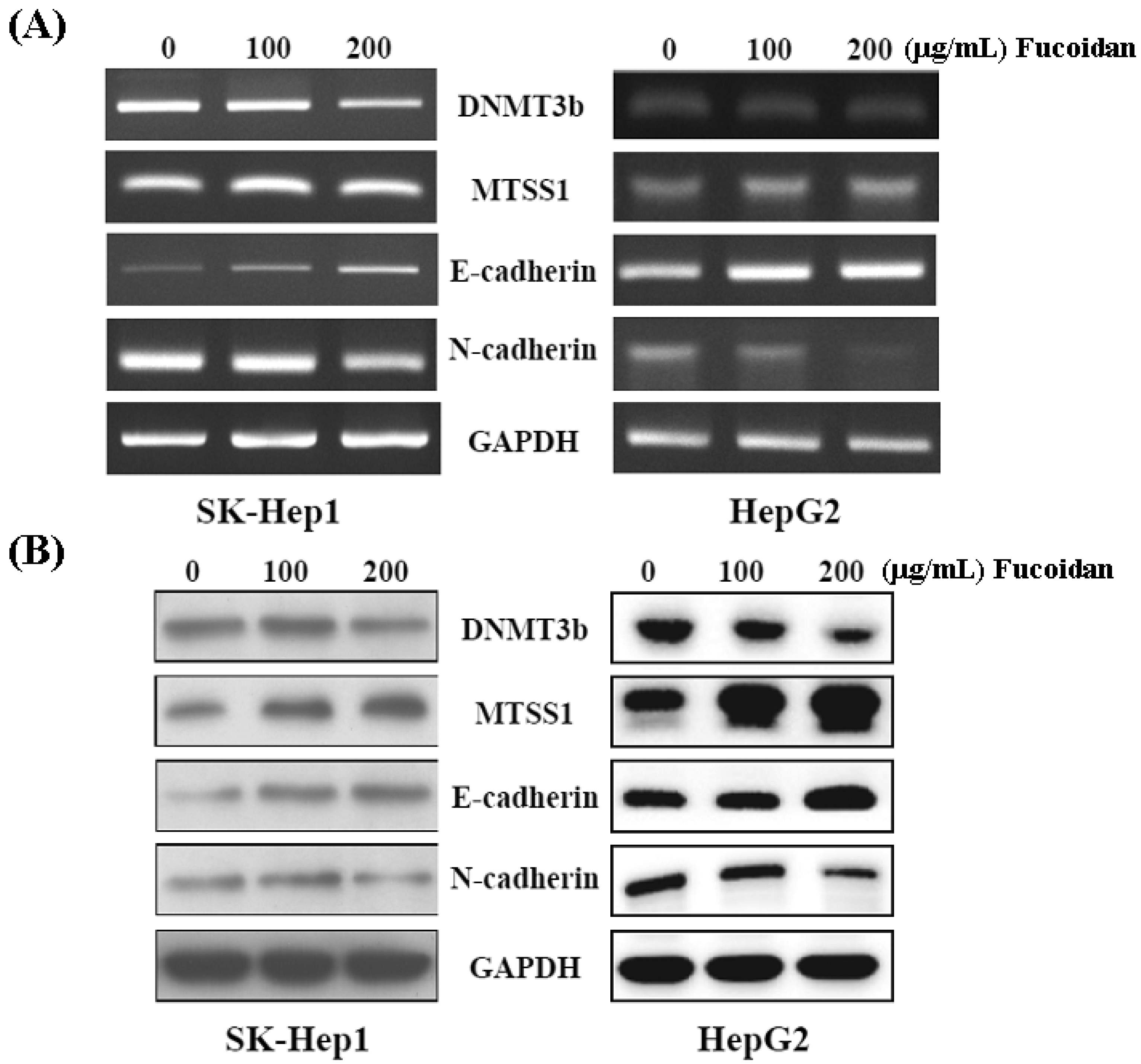
2.5. Fucoidan Increases Expression of Metastasis Suppressor 1 (MTSS1), a Novel Target of DNMT3B, Accompanied with EMT Inhibition
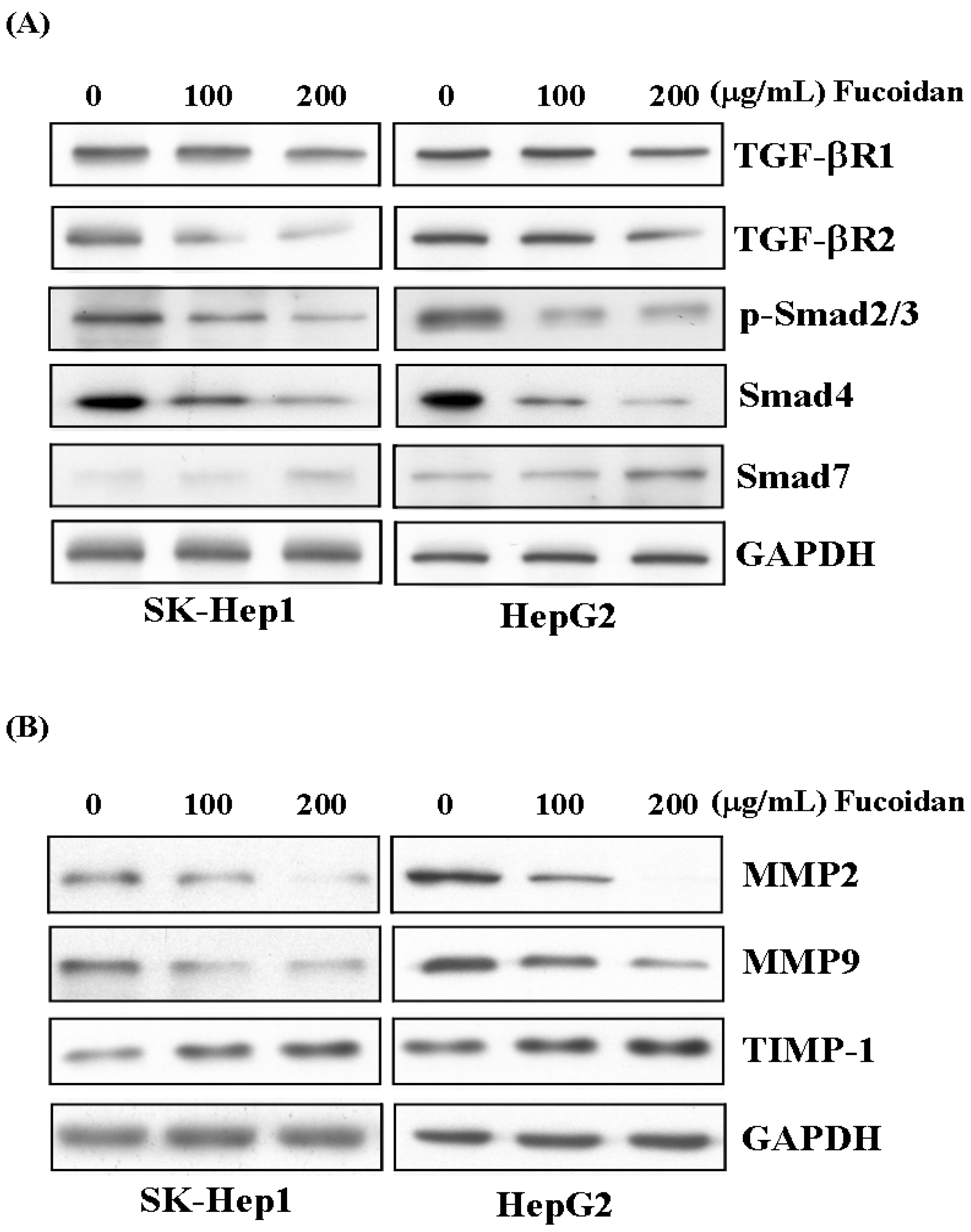
2.6. Fucoidan Suppresses TGF-β Signaling Pathway of HCC Cells and Prevents Extracellular Matrix Degradation
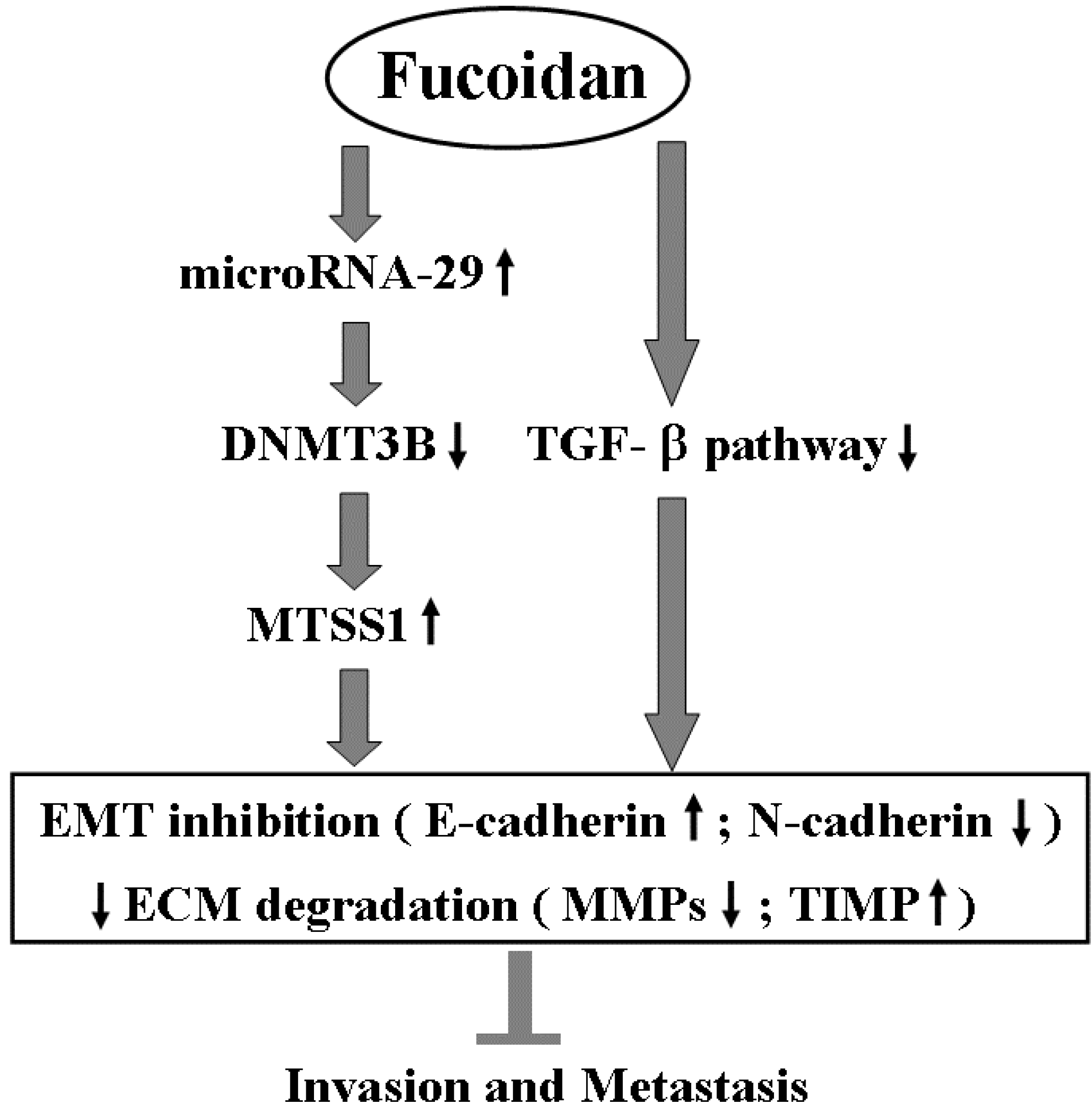
3. Discussion
4. Experimental Section
4.1. Reagents
4.2. Cell Culture
4.3. Cell Viability by MTS Assay
4.4. Colony Formation Assay
4.5. Invasion Assay
4.6. RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
| DNMT3B | Forward: AGGGAAGACTCGATCCTCGTC |
| Reverse: GTGTGTAGCTTAGCAGACTGG | |
| MTSS1 | Forward: CAGTCCCAGCTTCGGACAAC |
| Reverse: TGAGAGCAGATCCAATCTCCC | |
| E-cadherin | Forward: CGAGAGCTACACGTTCACGG |
| Reverse: GGGTGTCGAGGGAAAAATAGG | |
| N-cadherin | Forward: TCAGGCGTCTGTAGAGGCTT |
| Reverse: ATGCACATCCTTCGATAAGACTG | |
| GAPDH | Forward: GGAGCGAGATCCCTCCAAAAT |
| Reverse: GGCTGTTGTCATACTTCTCATGG | |
| miR-29b | Forward: TGGTTTCATATGGTGGTTTA |
| Reverse: ATAACCGATTTCAGATGGTG | |
| U6 | Forward: GTGCTCGCTTCGGCAGCACATATAC |
| Reverse: AAAAATATGGAACGCTTCACGAATTTG |
4.7. MicroRNA Profiling
4.8. Quantitative RT-PCR
4.9. MicroRNA Mimic and Construct of DNMT3B 3′-UTR Vectors
4.10. Transfection and Luciferase Reporter Assay
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, Z.Z.; Hsu, C.H.; Hsu, C.; Shao, Y.Y.; Cheng, A.L. Clinical trials in hepatocellular carcinoma: An update. Liver Cancer 2013, 2, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Arii, S. Molecular targeted therapies in hepatocellular carcinoma. Semin. Oncol. 2012, 39, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.B.; Olsen, S.K.; Magun, A.; Brown, R.S., Jr. Sorafenib: Where do we go from here? Hepatology 2010, 52, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Braconi, C.; Henry, J.C.; Kogure, T.; Schmittgen, T.; Patel, T. The role of microRNAs in human liver cancers. Semin. Oncol. 2011, 38, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Lyra-González, I.; Flores-Fong, L.E.; González-García, I.; Medina-Preciado, D.; Armendáriz-Borunda, J. MicroRNAs dysregulation in hepatocellular carcinoma: Insights in genomic medicine. World J. Hepatol. 2015, 7, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Garzon, R.; Marcucci, G.; Croce, C.M. Targeting microRNAs in cancer: Rationale, strategies and challenges. Nat. Rev. Drug Discov. 2010, 9, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Croce, C.M. microRNA involvement in human cancer. Carcinogenesis 2012, 33, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Fang, J.H.; Yun, J.P. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010, 51, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Urban, G.W.; Bettermann, K. MicroRNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 2011, 53, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; den Boon, J.A.; Chen, I.H. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 5874–5878. [Google Scholar] [CrossRef] [PubMed]
- Cittelly, D.M.; Finlay-Schultz, J.; Howe, E.N. Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. Oncogene 2013, 32, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Liu, S.; Fabbri, M.; Liu, Z.; Heaphy, C.E.; Callegari, E.; Schwind, S.; Pang, J.; Yu, J.; Muthusamy, N.; et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood 2009, 113, 6411–6418. [Google Scholar] [CrossRef] [PubMed]
- Cicchini, C.; de Nonno, V.; Battistelli, C.; Cozzolino, A.M.; de Santis Puzzonia, M.; Ciafrè, S.A.; Brocker, C.; Gonzalez, F.J.; Amicone, L.; Tripodi, M. Epigenetic control of EMT/MET dynamics: HNF4α impacts DNMT3s through miRs-29. Biochim. Biophys. Acta 2015, 1849, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Guo, Q.; Nan, X.X.; Wang, Z.; Yin, Z.; Yi, L.; Wei, Y.B.; Gao, Y.L.; Zhou, K.Q.; Yang, J.R. Micro-ribonucleic acid 29b inhibits cell proliferation and invasion and enhances cell apoptosis and chemotherapy effects of cisplatin via targeting of DNMT3b and AKT3 in prostate cancer. OncoTargets Ther. 2015, 8, 557–565. [Google Scholar]
- Neumann, O.; Kesselmeier, M.; Geffers, R.; Pellegrino, R.; Radlwimmer, B.; Hoffmann, K.; Ehemann, V.; Schemmer, P.; Schirmacher, P.; Lorenzo Bermejo, J.; et al. Methylome analysis and integrative profiling of human HCCs identify novel protumorigenic factors. Hepatology 2012, 56, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Portela, A.; Sayols, S.; Battiston, C.; Hoshida, Y.; Méndez-González, J.; Imbeaud, S.; Letouzé, E.; Hernandez-Gea, V.; Cornella, H.; et al. DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology 2015, 61, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kanai, Y.; Sakamoto, M.; Saito, H.; Ishii, H.; Hirohashi, S. Overexpression of a splice variant of DNA methyltransferase 3b, DNMT3b4, associated with DNA hypomethylation on pericentromeric satellite regions during human hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 10060–10065. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, J.; Wang, Y.; Zhu, J.; Zhou, K.; Smith, N.; Zhan, X. Differential regulation of cortactin and N-WASP-mediated actin polymerization by missing in metastasis (MIM) protein. Oncogene 2005, 24, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, L.; Zhang, F.; Quan, Y.; Su, X.; Qiu, X.; Zhao, Z.; Kong, K.L.; Dong, S.; Song, Y.; et al. MTSS1, a novel target of DNA methyltransferase 3B, functions as a tumor suppressor in hepatocellular carcinoma. Oncogene 2012, 31, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- Phuah, N.H.; Nagoor, N.H. Regulation of microRNAs by natural agents: New strategies in cancer therapies. Biomed. Res. Int. 2014, 2014, 804510. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y. Fucoidan as a marine anticancer agent in preclinical development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Hwang, P.A.; Tseng, L.M.; Chen, R.H.; Tsao, S.M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFβ receptor degradation in breast cancer. Carcinogenesis 2013, 34, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Wu, Y.C.; Tsao, S.M.; Hwang, P.A.; Shih, Y.W.; Hsu, J. Fucoidan inhibition of lung cancer in vivo and in vitro: Role of the Smurf2-dependent ubiquitin proteasome pathway in TGFβ receptor degradation. Oncotarget 2014, 15, 7870–7885. [Google Scholar] [CrossRef]
- Zhu, C.; Cao, R.; Zhang, S.X.; Man, Y.N.; Wu, X.Z. Fucoidan inhibits the growth of hepatocellular carcinoma independent of angiogenesis. Evid. Based Complement. Altern. Med. 2013, 2013, 692549. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.H.; Zhou, H.C.; Zeng, C.; Yang, J.; Liu, Y.; Huang, X.; Zhang, J.P.; Guan, X.Y.; Zhuang, S.M. MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology 2011, 54, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, R.; Chiyomaru, T.; Enokida, H.; Inoguchi, S.; Ishihara, T.; Matsushita, R.; Goto, Y.; Fukumoto, I.; Nakagawa, M.; Seki, N. Tumour-suppressive microRNA-29s directly regulate LOXL2 expression and inhibit cancer cell migration and invasion in renal cell carcinoma. FEBS Lett. 2015, 589, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.C.; Bruche, S.; Spence, H.J.; Braga, V.M.; Machesky, L.M. Mtss1 promotes cell-cell junction assembly and stability through the small GTPase Rac1. PLoS ONE 2012, 7, e31141. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Guo, Q.; Fu, F.J.; Wang, Z.; Yin, Z.; Wei, Y.B.; Yang, J.R. The role of miR-29b in cancer: Regulation, function, and signaling. OncoTargets Ther. 2015, 8, 539–548. [Google Scholar]
- Steele, R.; Mott, J.L.; Ray, R.B. MBP-1 upregulates miR-29b that represses Mcl-1, collagens, and matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, X.; Tu, Y.; Zheng, S.; Long, J.; Li, S.; Qi, C.; Xie, X.; Zhang, H.; Zhang, Y. MicroRNA-29b attenuates non-small cell lung cancer metastasis by targeting matrix metalloproteinase 2 and PTEN. J. Exp. Clin. Cancer Res. 2015, 34, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, C.; Lin, Z.; Guo, Y.; Shi, L.; Dong, P.; Lu, Z.; Gao, S.; Liao, Y.; Chen, B.; et al. Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation—A novel mechanism suppressing liver fibrosis. FEBS J. 2014, 281, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Ru, P.; Steele, R.; Newhall, P.; Phillips, N.J.; Toth, K.; Ray, R.B. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol. Cancer Ther. 2012, 11, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chung, D.; Shin, I.S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Hsu, W.L.; Hwang, P.A.; Chou, T.C. Low molecular weight fucoidan inhibits tumor angiogenesis through downregulation of HIF-1/VEGF signaling under hypoxia. Mar. Drugs 2015, 13, 4436–4451. [Google Scholar] [CrossRef] [PubMed]
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.-D.; Yao, C.-J.; Chow, J.-M.; Chang, C.-L.; Hwang, P.-A.; Chuang, S.-E.; Whang-Peng, J.; Lai, G.-M. Fucoidan Elevates MicroRNA-29b to Regulate DNMT3B-MTSS1 Axis and Inhibit EMT in Human Hepatocellular Carcinoma Cells. Mar. Drugs 2015, 13, 6099-6116. https://doi.org/10.3390/md13106099
Yan M-D, Yao C-J, Chow J-M, Chang C-L, Hwang P-A, Chuang S-E, Whang-Peng J, Lai G-M. Fucoidan Elevates MicroRNA-29b to Regulate DNMT3B-MTSS1 Axis and Inhibit EMT in Human Hepatocellular Carcinoma Cells. Marine Drugs. 2015; 13(10):6099-6116. https://doi.org/10.3390/md13106099
Chicago/Turabian StyleYan, Ming-De, Chih-Jung Yao, Jyh-Ming Chow, Chia-Lun Chang, Pai-An Hwang, Shuang-En Chuang, Jacqueline Whang-Peng, and Gi-Ming Lai. 2015. "Fucoidan Elevates MicroRNA-29b to Regulate DNMT3B-MTSS1 Axis and Inhibit EMT in Human Hepatocellular Carcinoma Cells" Marine Drugs 13, no. 10: 6099-6116. https://doi.org/10.3390/md13106099
APA StyleYan, M.-D., Yao, C.-J., Chow, J.-M., Chang, C.-L., Hwang, P.-A., Chuang, S.-E., Whang-Peng, J., & Lai, G.-M. (2015). Fucoidan Elevates MicroRNA-29b to Regulate DNMT3B-MTSS1 Axis and Inhibit EMT in Human Hepatocellular Carcinoma Cells. Marine Drugs, 13(10), 6099-6116. https://doi.org/10.3390/md13106099