Redox Status and Neuro Inflammation Indexes in Cerebellum and Motor Cortex of Wistar Rats Supplemented with Natural Sources of Omega-3 Fatty Acids and Astaxanthin: Fish Oil, Krill Oil, and Algal Biomass
Abstract
:1. Introduction
2. Results
2.1. Cerebellum
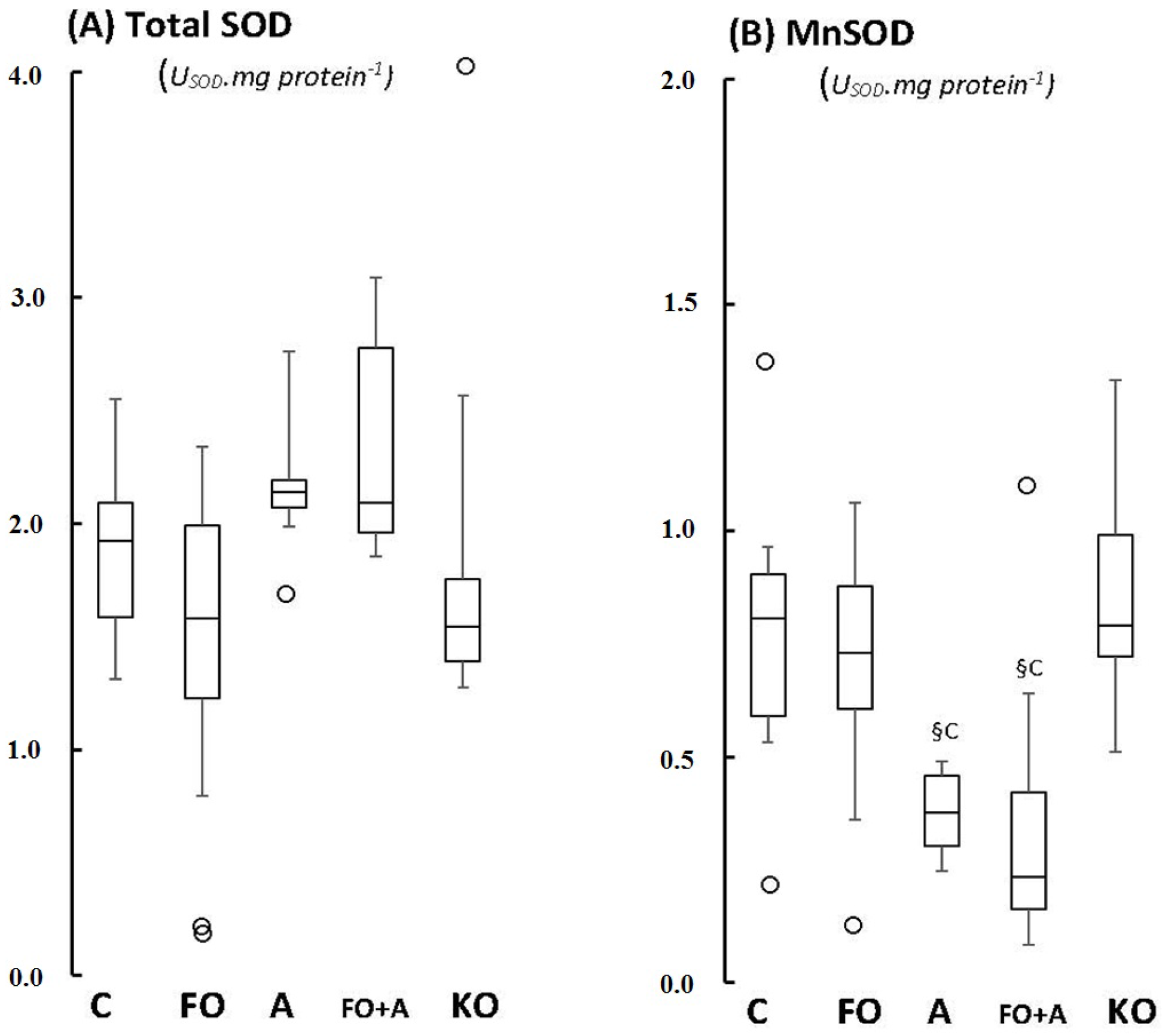

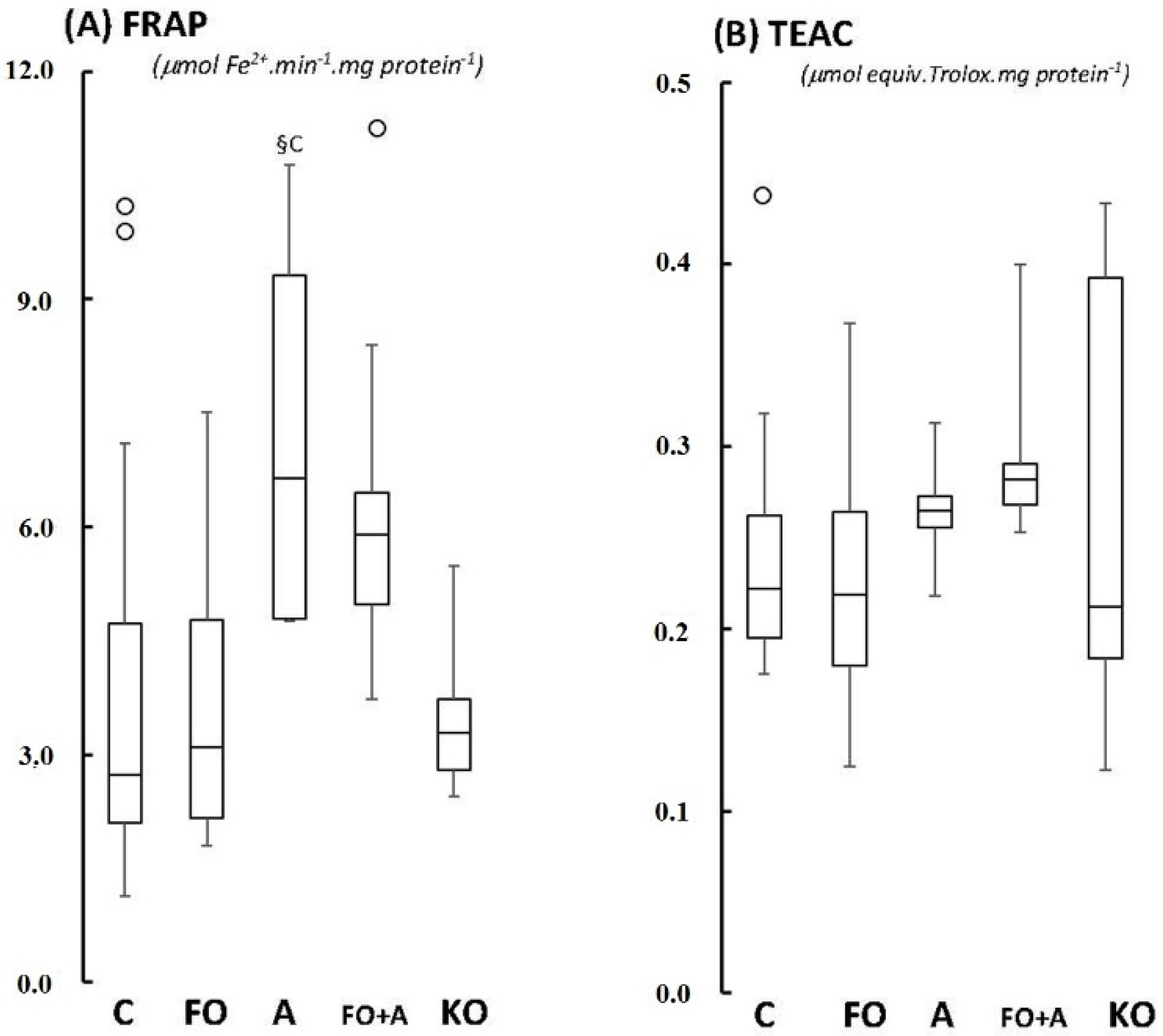
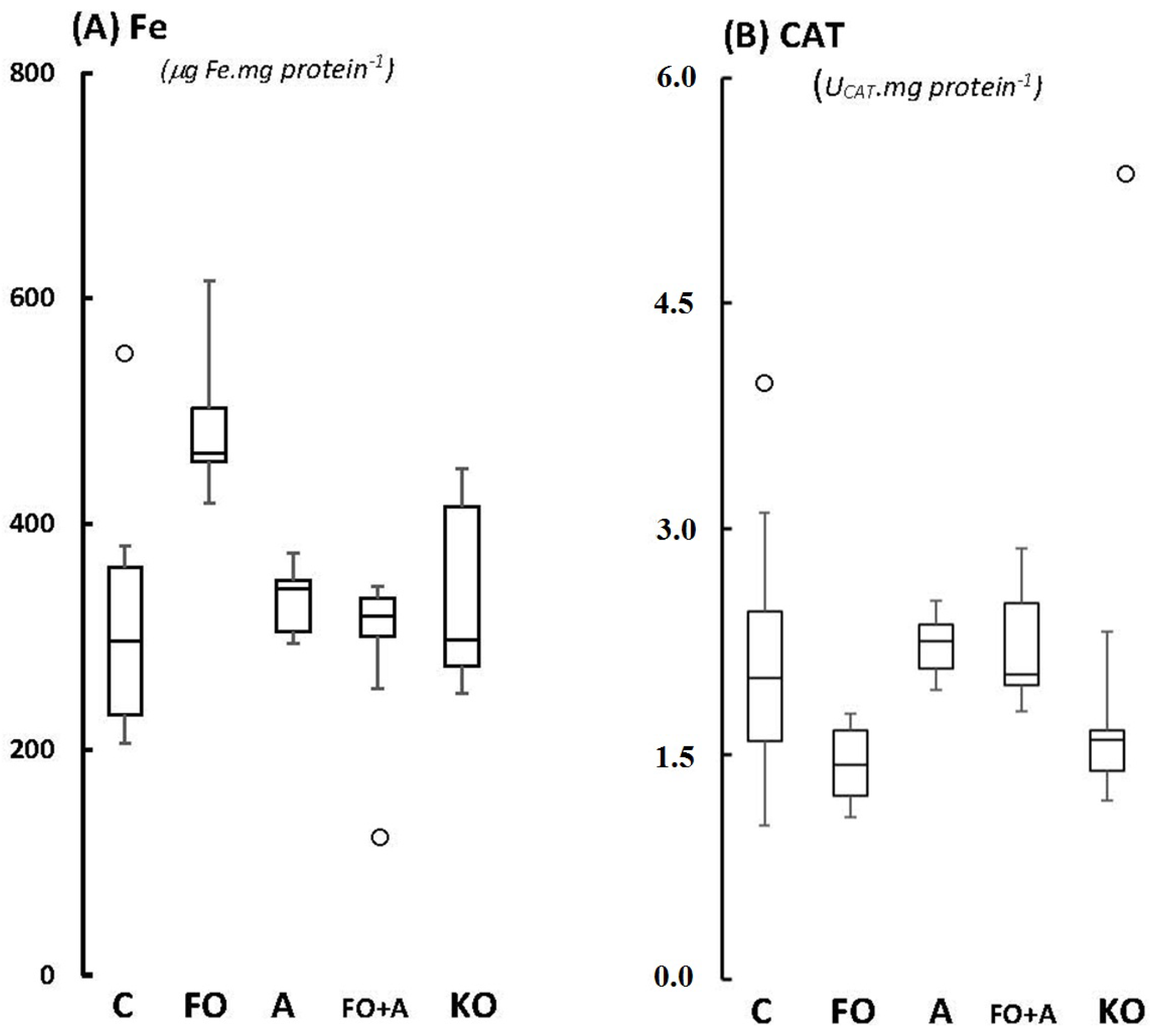
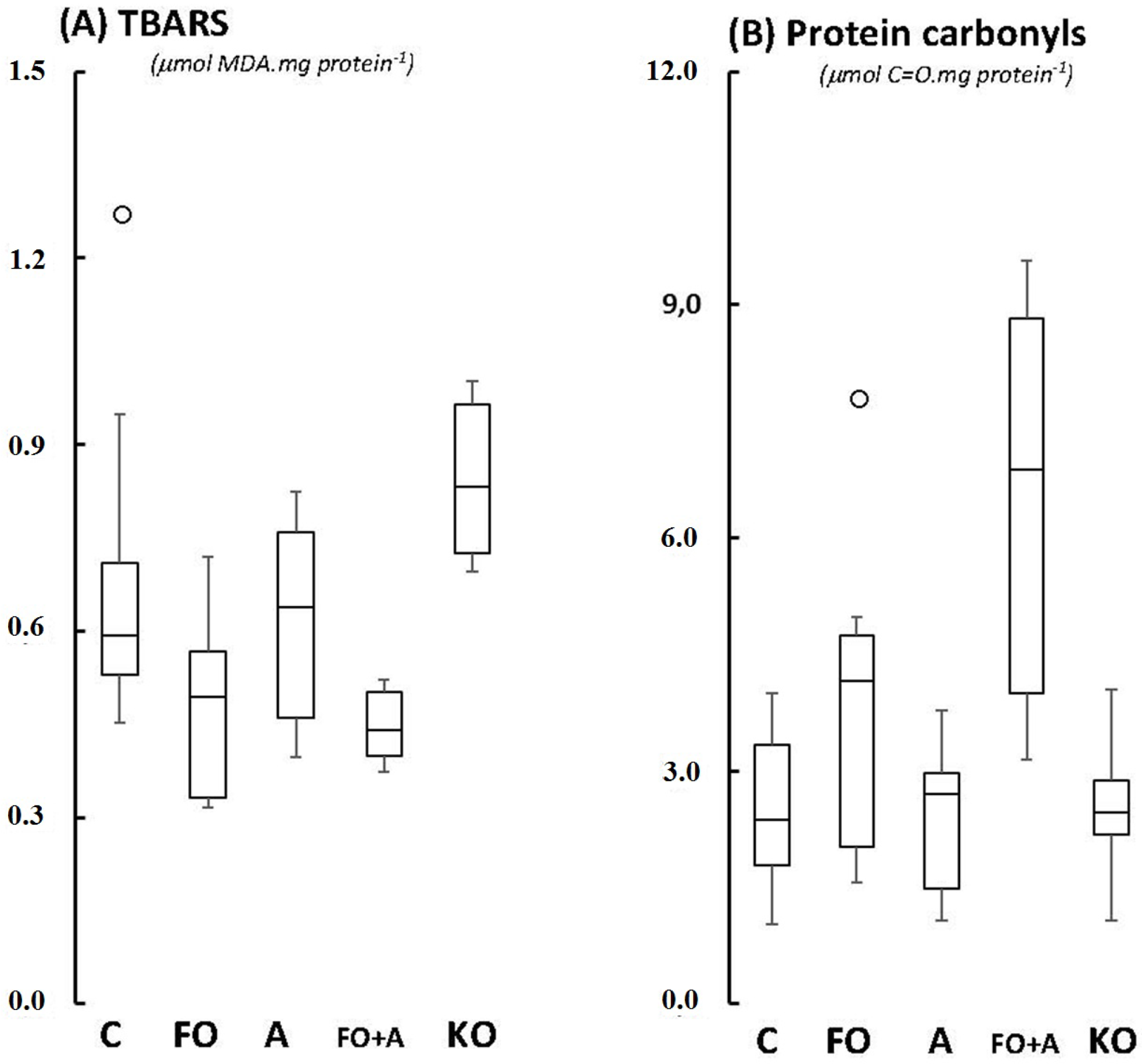
2.2. Motor Cortex (Molecular and Granular Layers) and Corpus Callosum
| Parameters a | Control | KO | p (t-Student’s) |
|---|---|---|---|
| GSH (µmoL/mg protein) | 71.1 ± 28.6 | 48.4 ± 9.9 | 0.085 |
| GSSG (µmoL/mg protein) | 39.2 ± 3.9 | 53.5 ± 26.0 | 0.180 |
| Reducing power (A.U.) | 0.464 ± 0.070 | 0.334 ± 0.122 | 0.317 |
| VEGF (pg/mg protein) | 39.3 ± 8.3 | 37.5 ± 12.5 | 0.284 |
| l-SEL (pg/mg protein) | 67.9 ± 65.0 | 52.2 ± 24.7 | 0.295 |
| CINC1 (ng/mg protein) | 3.76 ± 0.84 | 5.40 ± 3.49 | 0.060 |
| MIP1a (ng/mg protein) | 15.2 ± 5.3 | 12.8 ± 3.5 | 0.491 |
| TNF-α (ng/mg protein) | 17.5 ± 8.4 | 15.0 ± 2.2 | 0.371 |
| IL-6 (ng/mg protein) | 1.71 ± 0.91 | 1.32 ± 0.41 | 0.178 |
| IL-1β (ng/mg protein) | 1.30 ± 0.81 | 1.18 ± 0.47 | 0.388 |
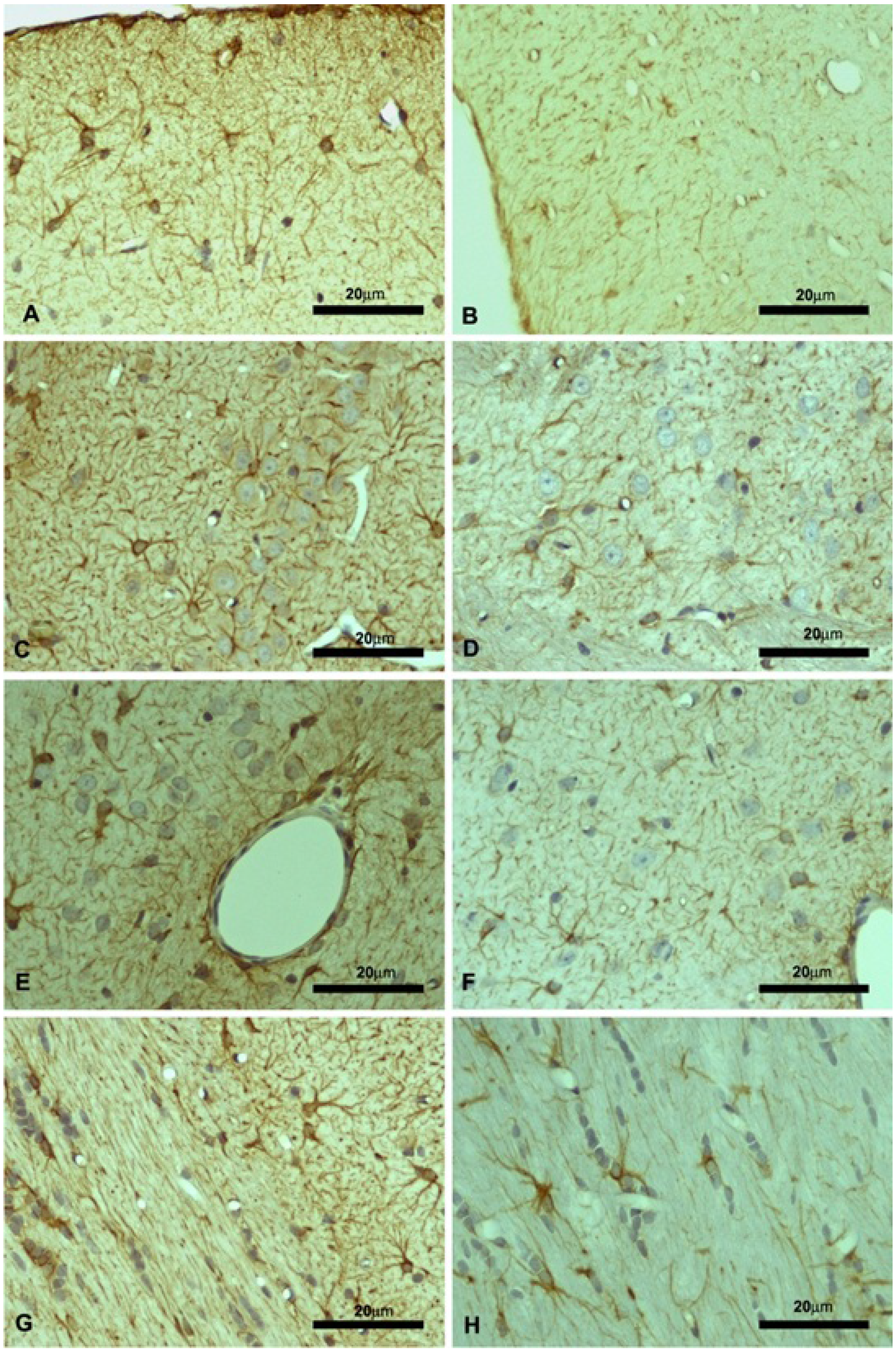
| Control | KO | |||||
|---|---|---|---|---|---|---|
| Area a | Mol. Layer | Gran. Layer | C. callosum | Mol. Layer | Gran. Layer | C. callosum |
| 1 | 5481.6 | 4391.7 | 4928.8 | 8435.5 | 6582.6 | 8374.7 |
| 2 | 4932.7 | 3837.9 | 3978.5 | 7345.4 | 7543.1 | 7945.5 |
| 3 | 4637.7 | 3291.8 | 4185.6 | 7581.5 | 6908.4 | 7531.9 |
| 4 | 4147.2 | 4317.8 | 3281.7 | 8190.2 | 6683.8 | 7435.8 |
| 5 | 3184.6 | 3928.8 | 4921.8 | 8284.7 | 7385.6 | 8528.6 |
| 6 | 4841.1 | 4193.8 | 3826.9 | 7858.5 | 7483.5 | 6836.7 |
| 7 | 3802.6 | 4928.8 | 4167.8 | 7593.0 | 6538.5 | 6638.5 |
| 8 | 4475.9 | 5102.3 | 3475.9 | 7395.7 | 7438.5 | 7578.9 |
| 9 | 3179.5 | 4294.9 | 4201.1 | 8193.6 | 8583.6 | 7538.6 |
| 10 | 3548.2 | 4291.7 | 3739.7 | 8530.5 | 8147.7 | 6625.8 |
| Mean (±SD) | 4223.1 (±782.5) | 4258.0 (±517.4) | 4070.8 (±543.0) | 7940.9 § (±440.4) | 7329.5 § (±675.3) | 7503.5 § (±665.1) |
3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Natural Sources of (n-3)/PUFAs and Astaxanthin
4.3. Animals and Supplementation Protocols
- (i)
- CONTROL: 0 mg EPA/kg BW + 0 mg DHA/kg BW + 0 mg ASTA/kg BW;
- (ii)
- FO: 10 mg EPA/kg BW + 7 mg DHA/kg BW + 0 mg ASTA/kg BW;
- (iii)
- ASTA: 0 mg EPA/kg BW + 0 mg DHA/kg BW + 1 mg ASTA/kg BW;
- (iv)
- FO + ASTA: 10 mg EPA/kg BW + 7 mg DHA/kg BW + 1 mg ASTA/kg BW;
- (v)
- KO: 10 mg EPA/kg BW + 4.7 mg DHA/kg BW + 0.0072 mg ASTA/kg BW.
4.4. Cerebellar Homogenates
4.4.1. Iron Content
4.4.2. Trolox-Equivalent and Ferric-Reducing Antioxidant Capacities
4.4.3. Antioxidant Enzyme Activities
4.4.4. Indexes of Lipid and Protein Oxidation
4.5. Motor Cortex and Corpus Callosum
4.5.1. Cytokines Determination
4.5.2. Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fatokun, A.A.; Stone, T.W.; Smith, R.A. Oxidative stress in neurodegeneration and available means of protection (Review). Front. Biosci. 2008, 13, 3288–3311. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H. Oxygen, antioxidants and brain dysfunction. Yonsei Med. J. 1993, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Langue, M.L.B.; Sultana, R. Involvements of the lipid peroxidation production, HNE, in the pathogenesis and progression of Alzheimer’s disease. Biochim. Biophys. Acta 2010, 180, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Whitton, P.S. Inflammation as a causative factor in the aethiology of Parkinson’s disease. Br. J. Pharmacol. 2007, 150, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Flood, P.M.; Hong, J.-S. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J. Neural Transm. 2010, 117, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Fonnum, F.; Lock, E.A. Cerebellum as a target for toxic substances. Toxicol. Lett. 2000, 112–113, 9–16. [Google Scholar] [CrossRef]
- Jolitha, A.B.; Subramanyam, M.V.; Asha Devi, S. Modification by vitamin E and exercise of oxidative stress in regions of aging rat brain: Studies on superoxide dismutase isoenzymes and protein oxidation status. Exp. Gerontol. 2006, 41, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; MacGarvey, U.; Kaufman, A.E.; Koontz, D.; Shoffner, J.M.; Wallace, D.C.; Beal, M.F. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann. Neurol. 1993, 34, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, I.; Gyte, A.J.; Mainwaring, G.; Widdowson, P.S.; Lock, E.A. Glutathione depletion in the liver and brain produced by 2-chloropropionic acid: Relevance to cerebellar granule cell necrosis. Arch. Toxicol. 1996, 70, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, T.; Verity, M.A. Oxidative mechanisms underlying methyl mercury neurotoxicity. Int. J. Dev. Neurosci. 1991, 9, 147–153. [Google Scholar] [CrossRef]
- Woods, E.A.; Hernandez, A.E.; Wagner, V.E.; Beilock, S.L. Expert athletes activate somatosensory and motor planning regions of the brain when passively listening to familiar sports sounds. Brain Cognit. 2014, 87, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Toldy, A.; Stadler, K.; Sasvári, M.; Jakus, J.; Jung, K.J.; Chung, H.Y.; Berkes, I.; Nyakas, C.; Radák, Z. The effect of exercise and nettle supplementation on oxidative stress markers in the rat brain. Brain Res. Bull. 2005, 65, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Hofer, T.; Rani, A.; Leeuwenburgh, C.; Foster, T.C. Comparison of lifelong and late life exercise on oxidative stress in the cerebellum. Neurobiol. Aging 2009, 30, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Poppe, S.C.; Bondan, E.F. Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, H. Update on marine omega-3 fatty acids: Management of dyslipidemia and current omega-3 treatment options. Atherosclerosis 2013, 230, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Mattei, R.; Polotow, T.G.; Vardaris, C.V.; Guerra, B.A.; Leite, J.R.; Otton, R.; Barros, M.P. Astaxanthin limits fish oil-related oxidative insult in the anterior forebrain of Wistar rats: Putative anxiolytic effects? Pharmacol. Biochem. Behav. 2011, 99, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.; Calviello, G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem. Res. Toxicol. 2011, 24, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Pinto, E.; Colepicolo, P.; Pedersén, M. Astaxanthin and peridinin inhibit oxidative damage in Fe2+-loaded liposomes: Scavenging oxyradicals or changing membrane permeability? Biochem. Biophys. Res. Commun. 2001, 288, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Otton, R.; Marin, D.P.; Bolin, A.P.; Santos, R.C.; Polotow, T.G.; Sampaio, S.C.; Barros, M.P. Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Bolin, A.P.; Macedo, R.C.; Marin, D.P.; Barros, M.P.; Otton, R. Astaxanthin prevents in vitro auto-oxidative injury in human lymphocytes. Cell Biol. Toxicol. 2010, 26, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Barros, M.P.; Poppe, S.C.; Souza-Junior, T.P. Putative benefits of micro alga astaxanthin on exercise and human health. Braz. J. Pharmacogn. 2011, 21, 283–289. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J.H. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipid Health Dis. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C.; Jaczynski, J.; Chen, Y.C. Krill for human consumption: Nutritional value and potential health benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Huang, M.C.; Diau, G.Y.; Boehm, G.; Nathanielsz, P.W.; Brenna, J.T. Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatr. Res. 2002, 51, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: Clinical findings and structural-functional synergies with cell membrane phospholipids. Altern. Med. Rev. 2007, 12, 207–227. [Google Scholar] [PubMed]
- Bunea, R.; El Farrah, K.; Deutsch, L. Evaluation of the effects of neptune krill oil on the clinical course of hyperlipidemia. Altern. Med. Rev. 2004, 9, 420–428. [Google Scholar] [PubMed]
- Batetta, B.; Griinari, M.; Carta, G.; Murru, E.; Ligresti, A.; Cordeddu, L.; Giordano, E.; Sanna, F.; Bisogno, T.; Uda, S.; et al. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J. Nutr. 2009, 139, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 2011, 46, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Pinton, S.; Brüning, C.A.; Sartori-Oliveira, C.E.; Prigol, M.; Nogueira, C.W. Therapeutic effect of organo-selenium dietary supplementation in a sporadic dementia of Alzheimer’s type model in rats. J. Nutr. Biochem. 2013, 24, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.M.; Tonin, F.S.; Barbiero, J.; Zaminelli, T.; Boschen, S.L.; Andreatini, R.; Da Cunha, C.; Lima, M.M.S.; Vital, M.A.B.F. The nonsteroidal antiinflammatory drug piroxicam reverses the onset of depressive-like behavior in 6-OHDA animal model of Parkinson’s disease. Neuroscience 2015, 300, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Vays, V.B.; Eldarov, C.M.; Vangely, I.M.; Kolosova, N.G.; Bakeeva, L.E.; Skulachev, V.P. Antioxidant SkQ1 delays sarcopenia-associated damage of mitochondrial ultrastructure. Aging 2014, 6, 140–148. [Google Scholar] [PubMed]
- Navarro, A.; Boveris, A. Brain mitochondrial dysfunction in aging, neurodegeneration, and Parkinson’s disease. Front. Aging Neurosci. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Osawa, T. Astaxanthin protects neuronal cells against oxidative damage and is a potent candidate for brain food. Forum Nutr. 2009, 61, 129–135. [Google Scholar] [PubMed]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2011, 105, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, C.S.; Lee, Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011, 49, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem. Biophys. Res. Commun. 2008, 366, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Zhang, J.; Reinhart, G.A.; Chew, B.P. Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dogs. J. Anim. Sci. 2013, 91, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Glowinski, J.; Iversen, L.L. Regional studies of catecholamines in the rat brain. I. The disposition of 3H-norepinephrine 3H-dopamine and 3H-DOPA in various regions of the brain. J. Neurochem. 1966, 13, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, S.; Tyagi, E.; Agrawal, R.; Singh, M.P.; Nath, C. A comparative study on oxidative stress induced by LPS and rotenone in homogenates of rat brain regions. Environ. Toxicol. Pharmacol. 2009, 27, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Gupta, S.; Joshi, N.; Sharma, S.; Singh, S. Astrocyte activation and neurotoxicity: A study in different rat brain regions and in rat C6 astroglial cells. Environ. Toxicol. Pharmacol. 2015, 40, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Inden, M.; Miyamura, A.; Kakimura, J.; Taniguchi, T.; Shimohama, S. Possible involvement of both mitochondria- and endoplasmic reticulum-dependent caspase pathways in rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 2002, 333, 25–28. [Google Scholar] [CrossRef]
- Wang, X.J.; Xu, J.X. Possible involvement of Ca2+ signaling in rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 2005, 376, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Baba, A.; Matsuda, T. Astrocyte apoptosis: Implications for neuroprotection. Prog. Neurobiol. 2004, 72, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar]
- Forman, H.J.; Davies, K.J.A.; Ursini, F.; Mattson, M.P.; Cheng, A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006, 29, 632–639. [Google Scholar]
- De Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.M.; Drukarch, B.; Hoozemans, J.; van Horssen, J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Smythies, J. Redox aspects of signaling by catecholamines and their metabolites. Antioxid. Redox Signal. 2000, 2, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Paula-Lima, A.C.; Adasme, T.; Hidalgo, C. Contribution of Ca2+ release channels to hippocampal synaptic plasticity and spatial memory: Potential redox modulation. Antioxid. Redox Signal. 2014, 21, 892–914. [Google Scholar] [CrossRef] [PubMed]
- Tadic, V.; Prell, T.; Lautenschlaeger, J.; Grosskreutz, J. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kurimura, Y.; Sakamoto, Y.; Tsuji, Y. Selective extraction of astaxanthin and chlorophyll from the green alga Haematococcus pluvialis. Biotechnol. Tech. 1997, 11, 657–660. [Google Scholar] [CrossRef]
- Stewart, J.S.; Lignell, A.; Pettersson, A.; Elfving, E.; Soni, M.G. Safety assessment of astaxanthin-rich microalgae biomass: Acute and subchronic toxicity studies in rats. Food Chem. Toxicol. 2008, 46, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Miki, W.; Toriu, W.; Kondo, Y.; Murakami, M.; Konosu, S.; Satake, M.; Fujta, T. The composition of carotenoid pigments in the Antarctic krill Euphausia superba. Nippon Suisan Gakkaishi 1983, 49, 1411–1415. [Google Scholar] [CrossRef]
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- Bralet, J.; Schreiber, L.; Bouvier, C. Effects of acidosis and anoxia on iron delocalization from brain homogenates. Biochem. Pharmacol. 1992, 43, 979–983. [Google Scholar] [CrossRef]
- Van den Berg, R.; Haenen, G.R.M.M.; van den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brewer, K.J.; Murphy, J.W.R.; Petersen, J.D. Synthesis and characterization of monometallic and bimetallic mixed-ligand complexes of iron(II) containing 2,2′-bipyrimidine or 2,3-bis(2-pyridyl)pyrazine. Inorg. Chem. 1987, 26, 3376–3379. [Google Scholar] [CrossRef]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Mannervik, B. Glutathione peroxidase. Methods Enzymol. 1985, 113, 490–495. [Google Scholar] [PubMed]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Murphy, M.E.; Kehrer, J.P. Oxidation state of tissue thiol groups and content of protein carbonyl groups in chickens with inherited muscular dystrophy. Biochem. J. 1989, 260, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Leibovitz, B.E.; Tappel, A.L. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radic. Biol. Med. 1988, 4, 155–161. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polotow, T.G.; Poppe, S.C.; Vardaris, C.V.; Ganini, D.; Guariroba, M.; Mattei, R.; Hatanaka, E.; Martins, M.F.; Bondan, E.F.; Barros, M.P. Redox Status and Neuro Inflammation Indexes in Cerebellum and Motor Cortex of Wistar Rats Supplemented with Natural Sources of Omega-3 Fatty Acids and Astaxanthin: Fish Oil, Krill Oil, and Algal Biomass. Mar. Drugs 2015, 13, 6117-6137. https://doi.org/10.3390/md13106117
Polotow TG, Poppe SC, Vardaris CV, Ganini D, Guariroba M, Mattei R, Hatanaka E, Martins MF, Bondan EF, Barros MP. Redox Status and Neuro Inflammation Indexes in Cerebellum and Motor Cortex of Wistar Rats Supplemented with Natural Sources of Omega-3 Fatty Acids and Astaxanthin: Fish Oil, Krill Oil, and Algal Biomass. Marine Drugs. 2015; 13(10):6117-6137. https://doi.org/10.3390/md13106117
Chicago/Turabian StylePolotow, Tatiana G., Sandra C. Poppe, Cristina V. Vardaris, Douglas Ganini, Maísa Guariroba, Rita Mattei, Elaine Hatanaka, Maria F. Martins, Eduardo F. Bondan, and Marcelo P. Barros. 2015. "Redox Status and Neuro Inflammation Indexes in Cerebellum and Motor Cortex of Wistar Rats Supplemented with Natural Sources of Omega-3 Fatty Acids and Astaxanthin: Fish Oil, Krill Oil, and Algal Biomass" Marine Drugs 13, no. 10: 6117-6137. https://doi.org/10.3390/md13106117






