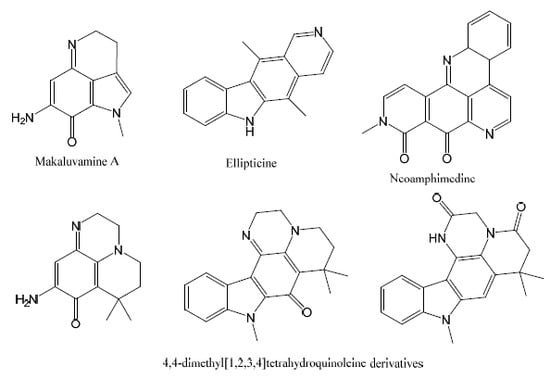
Design, Synthesis and Evaluation of New Marine Alkaloid-Derived Pentacyclic Structures with Anti-Tumoral Potency
Abstract
:1. Introduction
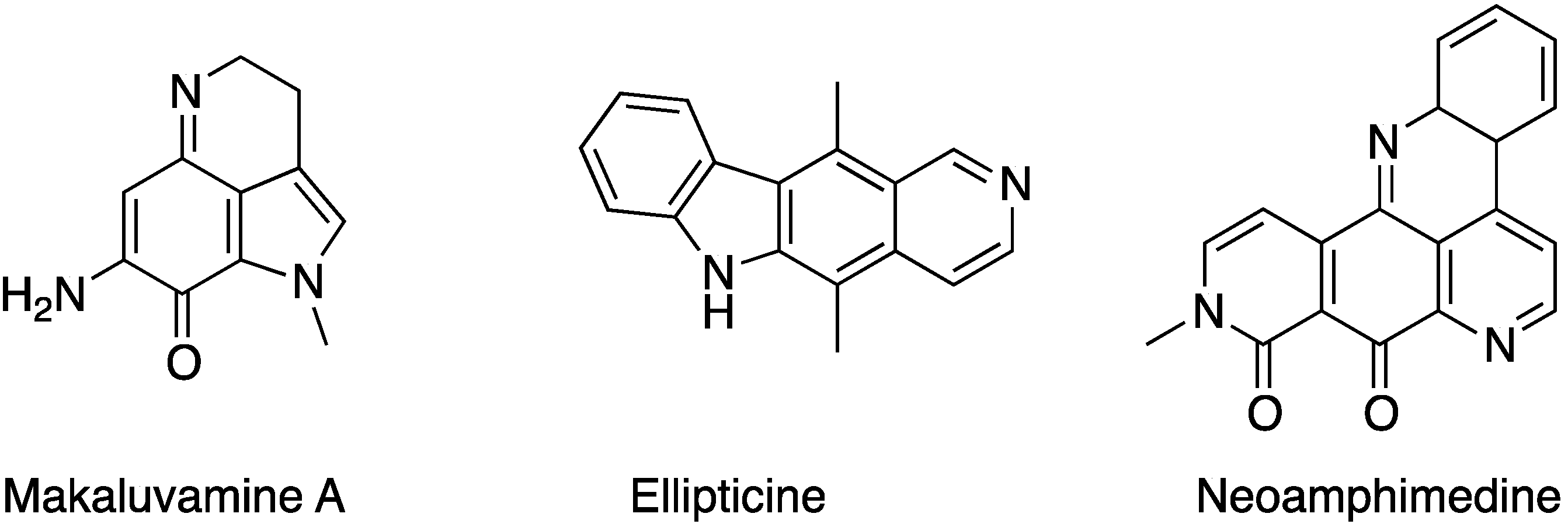
2. Results and Discussion
2.1. Analogue Design and Chemistry
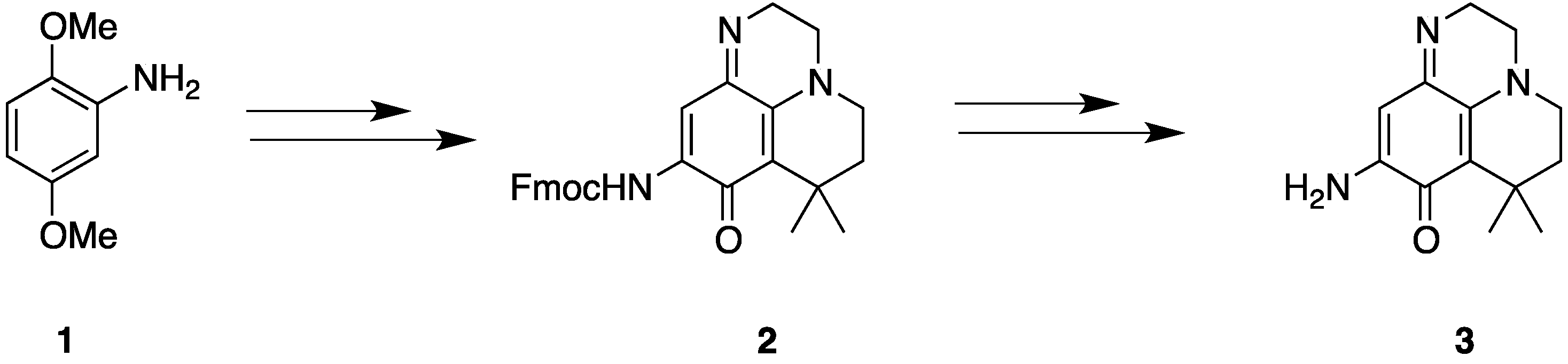
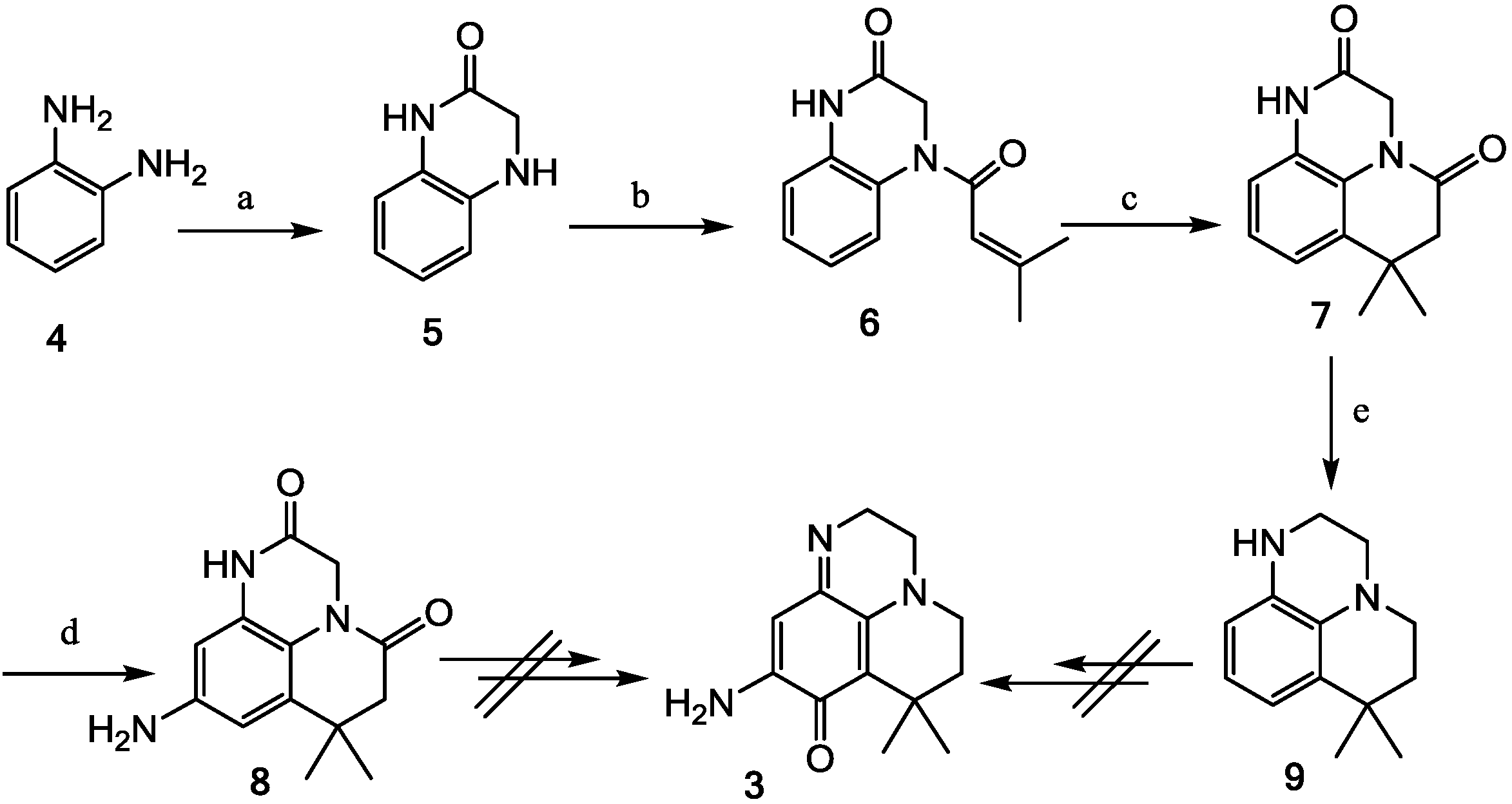
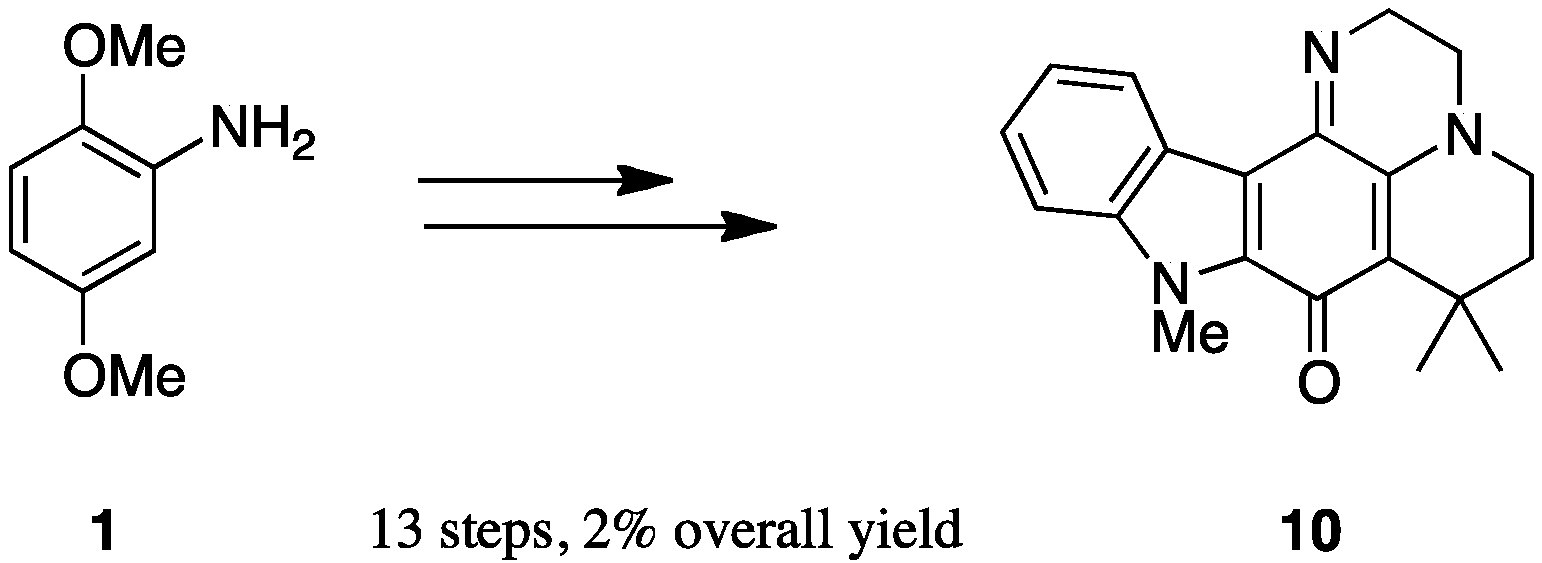
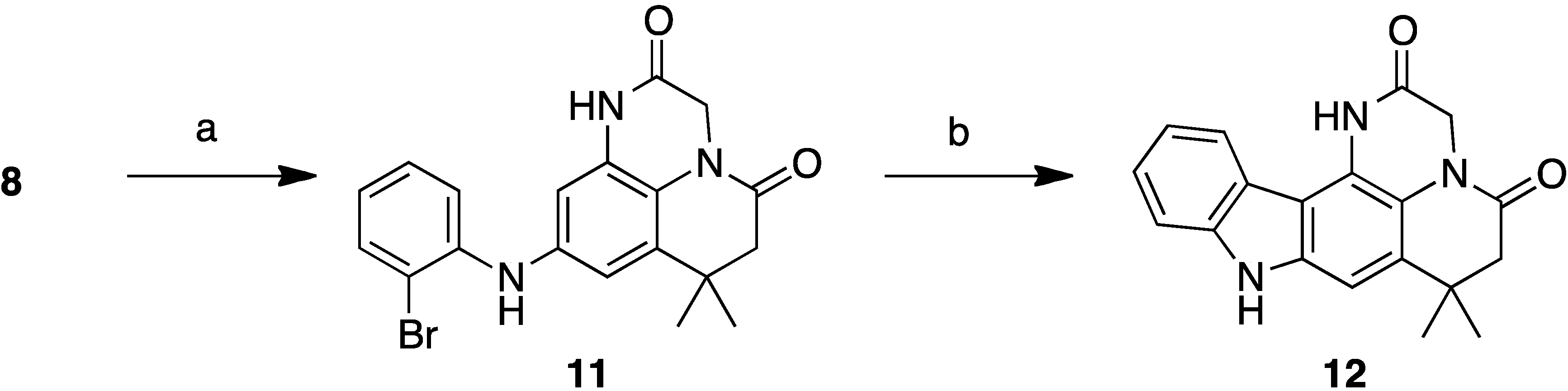
2.2. Bioactivities of the New Synthesized Analogues
2.2.1. In Vitro Cytotoxicity
| Cmpd | IC50 in μM | ||||||
|---|---|---|---|---|---|---|---|
| HUH-7 | CaCo-2 | MDA-MB-231 | HCT-116 | Pc-3 | NCI | Fib. Hum. | |
| 9 | 20 | 15 | 20 | 15 | 15 | 10 | >25 |
| 11 | 20 | 20 | >25 | 25 | 25 | >25 | 25 |
| 12 | 20 | 20 | 20 | 10 | 6 | 10 | 6 (50%) |
2.2.2. Topoisomerase II inhibition
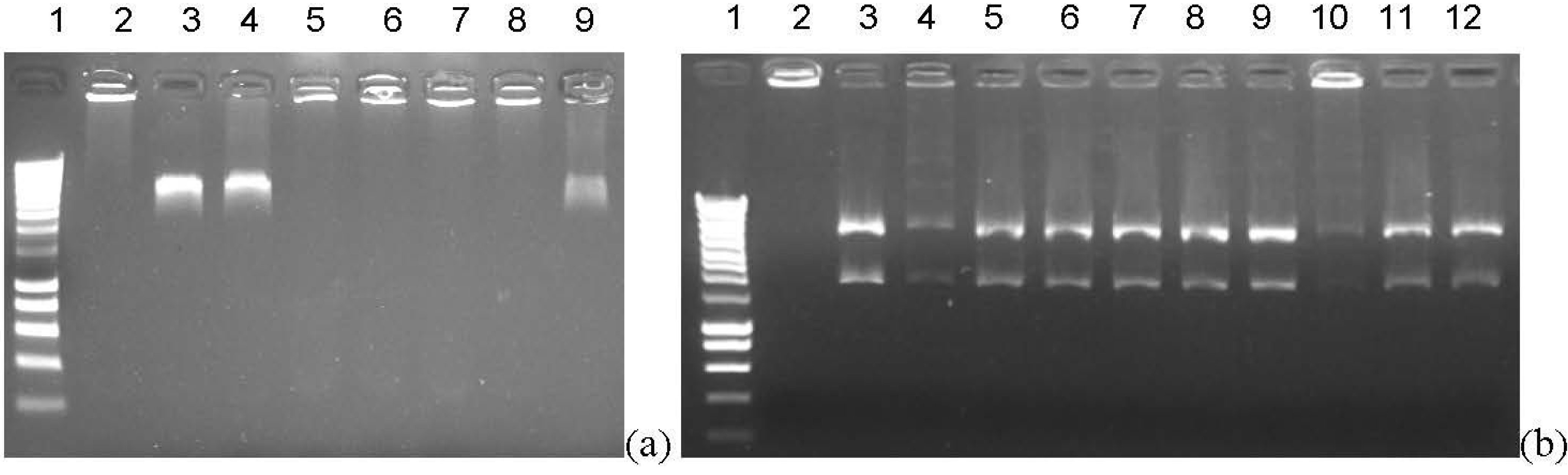
3. Experimental Section
3.1. Biology
3.1.1. Cytotoxicity Assay
3.1.2. Topoisomerase II Decatenation Assay
3.2. Chemistry
3.2.1. General Experimental Procedures
3.2.2. Synthesis of Compounds 5, 6, 7, 8, 9, 11 and 12
3,4-Dihydroquinoxalin-2(1H)-one (5)
4-(3-Méthylbut-2-ènoyl)-3,4-dihydroquinoxalin-2(1H)-one (6)
7,7-Diméthyl-6,7-dihydropyrido[1,2,3-de]quinoxaline-2,5(1H,3H)-dione (7)
9-Amino-7,7-diméthyl-6,7-dihydropyrido[1,2,3-de]quinoxaline-2,5(1H,3H)-dione (8)
7,7-Dimethyl-1,2,3,5,6,7-hexahydropyrido[1,2,3-de]quinoxaline (9)
9-(2-Bromophenylamino)-7,7-dimethyl-6,7-dihydropyrido[1,2,3-de]quinoxaline-2,5(1H,3H)-dione (11)
Compound 12
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Attmann, K.-H.; Gertsch, J. Anticancer drugs from nature—Natural products as a unique source of new microtubule-stabilizing agents. Nat. Prod. Rep. 2007, 24, 327–357. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Wink, M. Alkaloïds Biochemistry, Ecology and Medicinal Applications; Plenum Press: New York, NY, USA, 1998. [Google Scholar]
- Manske, R.H.F. The Alkaloids: Chemistry and Physiology; Academic Press: New York, NY, USA, 1981; Volumes 1–20. [Google Scholar]
- Aniszewski, T. Alakaloïds—Secret of Life, Alkaloïd Chemistry, Biological Significance, Applications and Ecological Role; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Meissner, C.F.W. Über Pflanzenalkalien: II. Über ein neues Pflanzenalkali (Alkaloid). J. Chem. Phys. 1819, 25, 377–381. [Google Scholar]
- Deane, F.M.; O’Sullivan, E.C.; Maguire, A.R.; Gilbert, J.; Sakoff, J.A.; McCluskey, A.; McCarthy, F.O. Synthesis and evaluation of novel ellipticines as potential anti-cancer agents. Org. Biomol. Chem. 2013, 11, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Ponder, J.; Yoo, B.H.; Abraham, A.D.; Li, Q.; Ashley, A.K.; Amerin, C.L.; Zhou, Q.; Reid, B.G.; Reigan, P.; Hromas, R.; et al. Neoamphimedine circumvents metnase-enhanced DNA topoisomerase II alpha activity through ATP-competitive inhibition. Mar. Drugs 2011, 9, 2397–2408. [Google Scholar] [CrossRef]
- Barrows, L.R.; Radisky, D.C.; Copp, B.R.; Swaffar, D.S.; Kramer, R.A.; Waters, R.L.; Ireland, C.M. Makaluvamines, marine natural products, are active anti-cancer agents and DNA topo II inibitors. Anti-Cancer Drug Des. 1993, 8, 333–347. [Google Scholar]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornprobst, J.M. Substances Naturelles D’origine Marine: Chimiodiversité, Pharmacodiversité, Biotechnologies; Éditions Tec & Doc Lavoisier: Paris, France, 2005; Volume 1, p. 1800. [Google Scholar]
- Nag, S.; Nadkarni, D.H.; Qin, J.-J.; Voruganti, S.; Nguyen, T.; Xu, S.; Wang, W.; Wang, H.; Velu, S.E.; Zhang, R. Anticancer activity and molecular mechanisms of action of makaluvamines and analogues. Mol. Cell. Pharmacol. 2012, 4, 69–81. [Google Scholar]
- West, L.M.; Northcote, P.T.; Battershill, C.N. Peloruside A: A potent cytotoxic macrolide isolated from the New Zealand marine sponge Mycale sp. J. Org. Chem. 2000, 65, 445–449. [Google Scholar] [CrossRef]
- Kokoshka, J.M.; Capson, T.L.; Holden, J.A.; Ireland, C.M.; Barrows, L.R. Differences in the topoisomerase I cleavage complexes formed by camptothecin and wakayin, a DNA-intercalating marine natural product. Anti-Cancer Drugs 1996, 7, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Pinkert, A.; Blunt, J.W.; Munro, M.H.G. Discorhabdin W, the first dimeric discorhabdin. J. Nat. Prod. 2005, 68, 1796–1798. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.W.; Harper, M.K.; Faulkner, D.J. Makaluvamines H–M and Damirone C from the pohnpeian sponge Zyzzya fuliginosa. J. Nat. Prod. 1995, 58, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; Blunt, J.W.; Munro, M.H.G. Discorhabdin D, an antitumor alkaloid from the sponges latrunculia brevis and Prianos sp. J. Org. Chem. 1988, 53, 4127–4218. [Google Scholar] [CrossRef]
- Guillard, J.; Bouclé, S. Synthesis of Analogues of Makaluvamine A. Heterocycles 2012, 85, 677. [Google Scholar] [CrossRef]
- TenBrink, R.E.; Im, W.B.; Sethy, V.H.; Tang, A.H.; Carter, D.B. Antagonist, partial agonist, and full agonist imidazo[1,5-a]quinoxaline amides and carbamates acting through the GABAA/Benzodiazepine receptor. J. Med. Chem. 1994, 37, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Guillard, J.; Bouclé, S. Synthesis of a New pyrido[3,2-b]carbazole as an ellipticine-makaluvamine hybrid. Synthesis 2011, 10, 1616–1620. [Google Scholar] [CrossRef]
- Yang, S.Y.; Jia, X.Z.; Feng, L.Y.; Li, S.Y.; An, G.S.; Ni, J.H.; Jia, H.T. Inhibition of topoisomerase II by 8-chloro-adenosine triphosphate induces DNA double-stranded breaks in 8-chloro-adenosine-exposed human myelocytic leukemia K562 cells. Biochem. Pharmacol. 2009, 77, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boucle, S.; Melin, C.; Clastre, M.; Guillard, J. Design, Synthesis and Evaluation of New Marine Alkaloid-Derived Pentacyclic Structures with Anti-Tumoral Potency. Mar. Drugs 2015, 13, 655-665. https://doi.org/10.3390/md13010655
Boucle S, Melin C, Clastre M, Guillard J. Design, Synthesis and Evaluation of New Marine Alkaloid-Derived Pentacyclic Structures with Anti-Tumoral Potency. Marine Drugs. 2015; 13(1):655-665. https://doi.org/10.3390/md13010655
Chicago/Turabian StyleBoucle, Sebastien, Celine Melin, Marc Clastre, and Jerome Guillard. 2015. "Design, Synthesis and Evaluation of New Marine Alkaloid-Derived Pentacyclic Structures with Anti-Tumoral Potency" Marine Drugs 13, no. 1: 655-665. https://doi.org/10.3390/md13010655