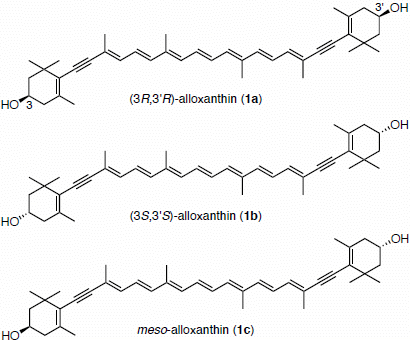
Synthesis of (3S,3′S)- and meso-Stereoisomers of Alloxanthin and Determination of Absolute Configuration of Alloxanthin Isolated from Aquatic Animals
Abstract
:1. Introduction
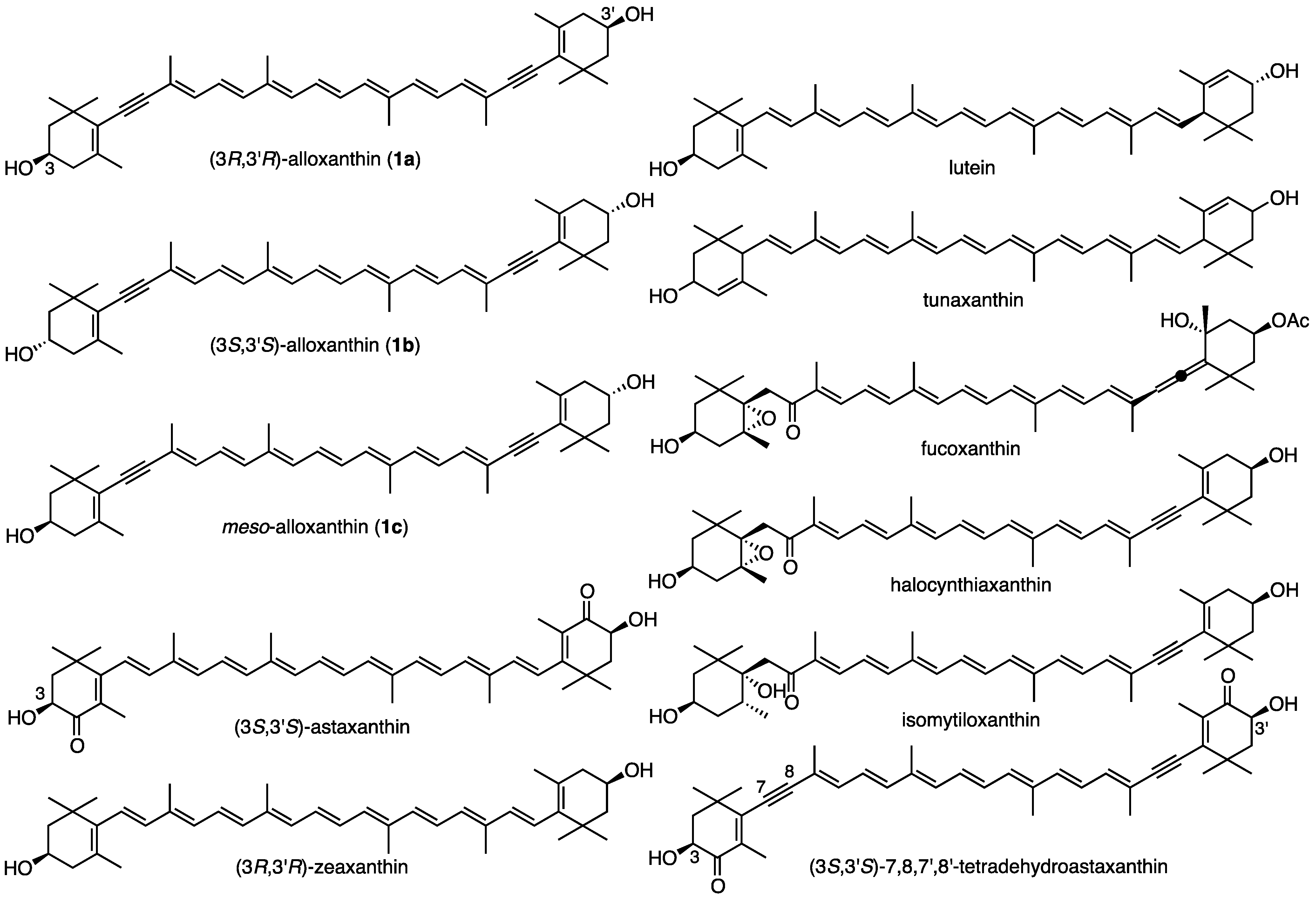
2. Results and Discussion
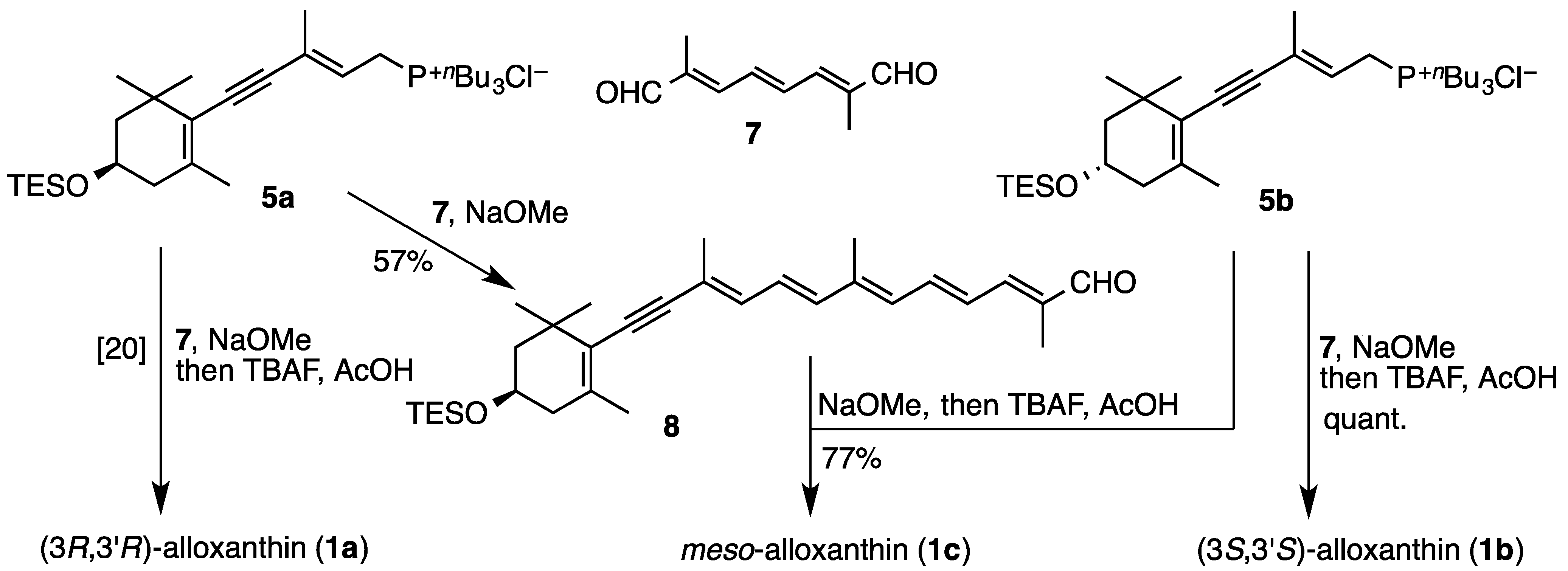
2.1. Synthesis of (3S,3′S)-Alloxanthin (1b) and meso-Alloxanthin (1c)

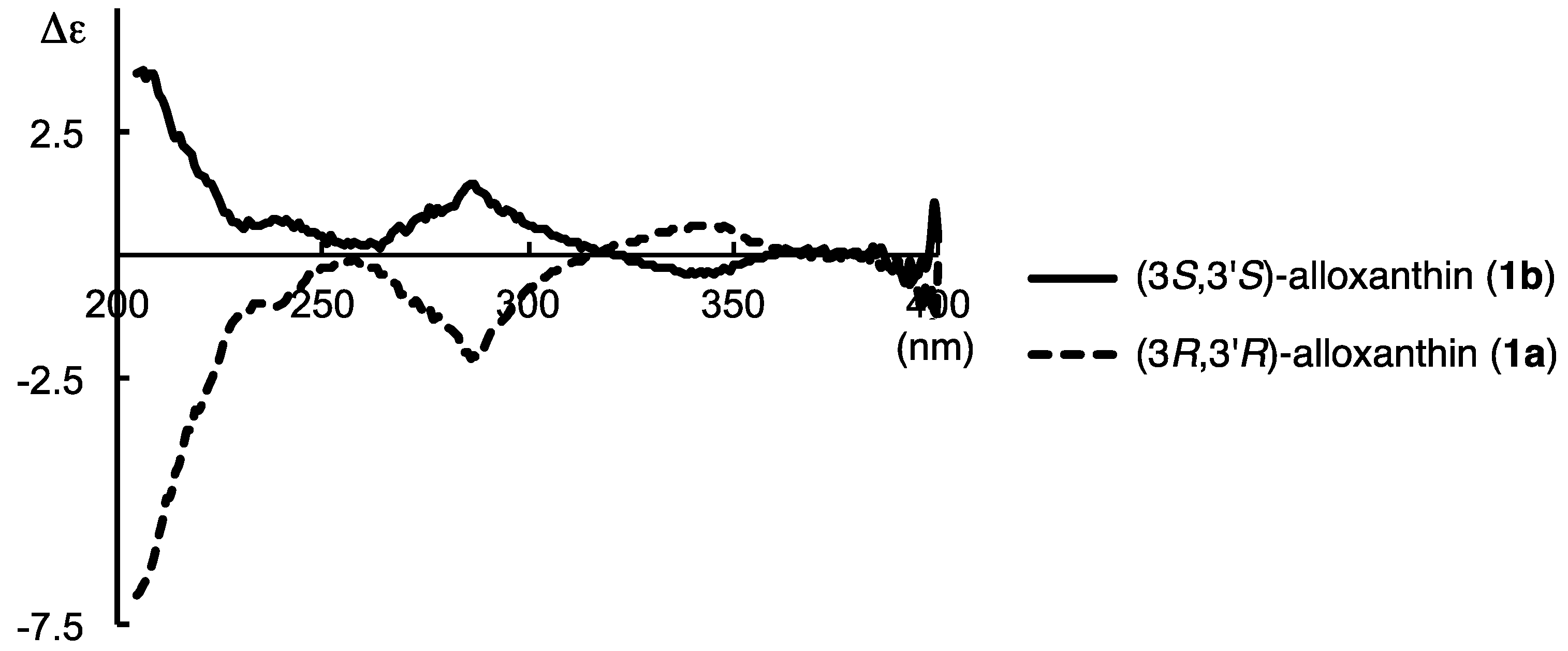
2.2. Determination of Absolute Configuration of Alloxanthin Isolated from Aquatic Animals by HPLC
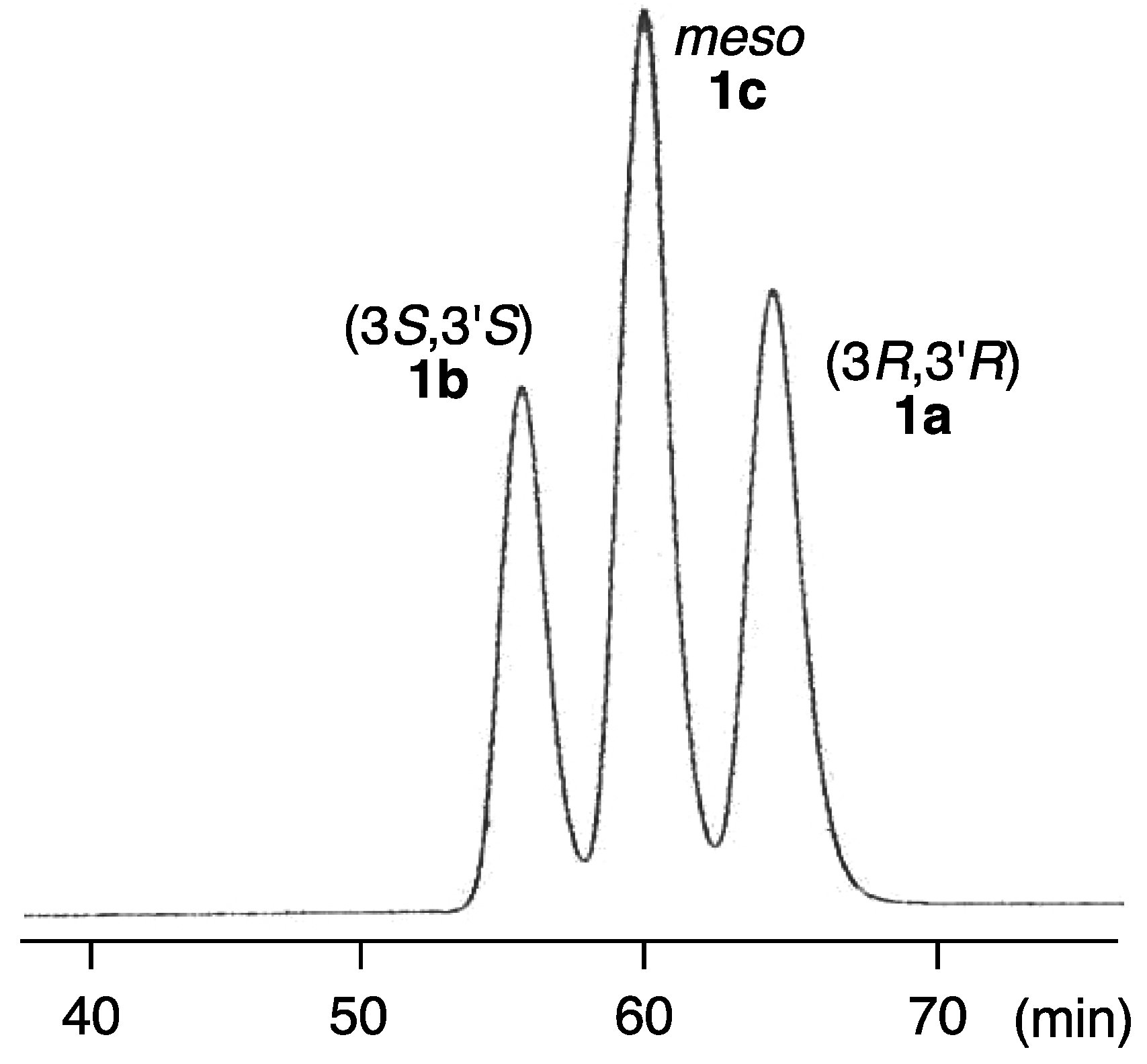
| Species | 3R,3′R | 3S,3′S | meso | |
|---|---|---|---|---|
| 1a | 1b | 1c | ||
| Shellfish | ||||
| Scallop | Mizuhopecten yessoensis | 100 | n.d. | n.d. |
| Oyster | Crassostrea gigas | 100 | n.d. | n.d. |
| Pacific pearl oyster | Pinctada margaritifera | 100 | n.d. | n.d. |
| Freshwater bivalves | Unio douglasiae | 100 | n.d. | n.d. |
| Tunicate | ||||
| Sea squirt | Halocynthia roretzi | 100 | n.d. | n.d. |
| Crustacean | ||||
| Lake shrimp | Palaemon paucidens | 53.7 | 9.6 | 36.7 |
| Fish | ||||
| Crucian carp | Carassius auratus grandoculis | 100 | n.d. | n.d. |
| Biwa goby | Gymnogobius isaza | 91.4 | 0.9 | 7.7 |
| Biwa trout | Oncorhynchus masou rhodurus | >99.9 | trace | trace |
| Catfish | Silurus asotus | 82.9 | 1.5 | 15.6 |
3. Experimental Section
3.1. General
3.2. Synthesis of (3S,3′S)-Alloxanthin (1b) and meso-Alloxanthin (1c)
3.3. Configurational Analysis of Natural Alloxanthin
3.3.1. Animal Materials
3.3.2. Isolation of Alloxanthin from Aquatic Animals
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haxo, F.T.; Fork, D.C. Photosynthetically active accessory pigments of cryptomonads. Nature 1959, 184, 1051–1052. [Google Scholar] [CrossRef]
- Mallams, A.K.; Waight, E.S.; Weedon, B.C.L.; Chapman, D.J.; Haxo, F.T.; Goodwin, T.W.; Thomas, B.M. A new class of carotenoids. Chem. Commun. 1967, 301–302. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Suzuki, Y. Biochemical studies of the ascidian, Cynthia rorezi variety drasche. IV Carotenoids in test. Tohoku J. Agric Res. 1959, 10, 397–407. [Google Scholar]
- Nishibori, K. Pigments of marine animlas—VIII. Carotenoids of some shellfish. Publ. Seto Mar. Biol. Lab. 1960, 8, 317–326. [Google Scholar]
- Campbell, S.A.; Mallams, A.K.; Waight, E.S.; Weedon, B.C.L. Pectenoxanthin, cynthiaxanthin, and a new acetylenic carotenoid, pectenolone. Chem. Commun. 1967, 941–942. [CrossRef]
- DeVille, T.E.; Hursthouse, M.B.; Russell, S.W.; Weedon, B.C.L. Absolute configuration of carotenoids. Chem. Commun. 1969, 1311–1312. [Google Scholar] [CrossRef]
- Bartlett, L.; Klyne, W.; Mose, W.P.; Scopes, P.M.; Galasko, G.; Mallams, A.K.; Weedon, B.C.L.; Szabolcs, J.; Toth, G. Optical rotatory dispersion of carotenoids. J. Chem. Soc. Perkin Trans. 1 1969, 2527–2544. [Google Scholar] [CrossRef]
- Hertzberg, S.; Partali, V.; Liaaen-Jensen, S. Animal carotenoids. 32. Carotenoids of Mytilus edulis (edible mussel). Acta Chem. Scand. 1988, B42, 495–503. [Google Scholar]
- Maoka, T.; Matsuno, T. Carotenoids of shellfishes—IX. Isolation and structural elucidation of three new acetylenic carotenoids from the Japanese sea mussel Mytilus coruscus. Nippon Suisan Gakkaishi 1988, 54, 1443–1447. [Google Scholar] [CrossRef]
- Maoka, T.; Tsushima, M.; Matsuno, T. New acetylenic carotenoids from the starfishes Asterina pectinifera and Asterias amurensis. Comp. Biochem. Physiol. 1989, 93B, 829–834. [Google Scholar]
- Matsuno, T.; Ookubo, M.; Nishizawa, T.; Shimizu, I. Carotenoids of sea squirts. I. New marine carotenoids, halocynthiaxanthin and mytiloxanthinone from Halocynthia roretzi. Chem. Pharm. Bull. 1984, 32, 4309–4315. [Google Scholar] [CrossRef]
- Ookubo, M.; Matsuno, T. Carotenoids of sea squirts—II. Comparative biochemical studies of carotenoids in sea squirts. Comp. Biochem. Physiol. 1985, 81B, 137–141. [Google Scholar]
- Matsuno, T.; Maoka, T.; Ikuno, Y. Comparative biochemical studies of carotenoids in fish—XXVII. Carotenoids in the eggs of three species of Cyprinidae. Comp. Biochem. Physiol. 1986, 83B, 335–337. [Google Scholar]
- Maoka, T.; Akiomoto, N. Structures of minor carotenoids from the Japanese common catfish, Silurus asotus. Chem. Phram. Bull. 2011, 59, 140–145. [Google Scholar] [CrossRef]
- Ronneberg, H.; Renstrom, B.; Aareskjold, K.; Liaaen-Jensen, S.; Vecchi, M.; Leuenberger, F.J.; Mȕller, R.K.; Mayer, H. Naturally occurrence of enantiomeric and meso-astaxanthin 1. Ex lobster eggs (Homarus gammarus). Helv. Chim. Acta 1980, 63, 711–715. [Google Scholar] [CrossRef]
- Matsuno, T.; Maoka, T.; Katsuyama, M.; Ookubo, M.; Katagiri, K.; Jimura, H. The occurrence of enantiomeric and meso-astaxanthin in aquatic animals. Nippon Suisan Gakkaishi 1984, 50, 1589–1592. [Google Scholar] [CrossRef]
- Maoka, T.; Arai, A.; Shimizu, M.; Matsuno, T. The first isolation of enantiomeric and meso-zeaxanthin in nature. Comp. Biochem. Physiol. 1986, 83B, 121–124. [Google Scholar]
- Matsuno, T.; Maoka, T.; Katsuyama, M.; Hirono, T.; Ikuno, Y.; Shimizu, M.; Komori, T. Comparative biochemical studies of carotenoids in fishes—XXIX. Isolation of new luteins, lutein F and lutein G from marine fishes. Comp. Biochem. Physiol. 1986, 85B, 77–80. [Google Scholar]
- Ikuno, Y.; Shimizu, M.; Koshino, Y.; Maoka, T.; Matsuno, T. Comparative biochemical studies of carotenoids in fishes—XXVII. Stereochemical investigation of carotenoids from yellow-tail rockfish Sebastes flavidus. Nippon Suisan Gakkaishi 1985, 51, 2033–2035. [Google Scholar] [CrossRef]
- Yamano, Y.; Chary, V.M.; Wada, A. Stereoselective total synthesis of the acetylenic carotenoids alloxanthin and triophaxanthin. Org. Biomol. Chem. 2012, 10, 4103–4108. [Google Scholar] [CrossRef]
- Leuenberger, H.G.W.; Boguth, W.; Widmer, E.; Zell, R. Synthesis of optically active natural carotenoids and structurally related compounds. I. Synthesis of the chiral key compound (4R,6R)-4-hydroxy-2,2,6-trimethylcyclohexanone. Helv. Chim. Acta 1976, 59, 1832–1849. [Google Scholar] [CrossRef]
- Partali, V.; Tangen, K.; Liaaen-Jensen, S. Carotenoids in food chain studies. III. Resorption and metabolic transformation of carotenoids in Mytilus edulis (edible mussel). Comp. Biochem. Physiol. 1989, 92B, 239–246. [Google Scholar]
- Liaaen-Jensen, S. Carotenoids in food chain. In Carotenoids Volume 3: Biosynthesis and Metabolism; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1998; pp. 359–371. [Google Scholar]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamano, Y.; Maoka, T.; Wada, A. Synthesis of (3S,3′S)- and meso-Stereoisomers of Alloxanthin and Determination of Absolute Configuration of Alloxanthin Isolated from Aquatic Animals. Mar. Drugs 2014, 12, 2623-2632. https://doi.org/10.3390/md12052623
Yamano Y, Maoka T, Wada A. Synthesis of (3S,3′S)- and meso-Stereoisomers of Alloxanthin and Determination of Absolute Configuration of Alloxanthin Isolated from Aquatic Animals. Marine Drugs. 2014; 12(5):2623-2632. https://doi.org/10.3390/md12052623
Chicago/Turabian StyleYamano, Yumiko, Takashi Maoka, and Akimori Wada. 2014. "Synthesis of (3S,3′S)- and meso-Stereoisomers of Alloxanthin and Determination of Absolute Configuration of Alloxanthin Isolated from Aquatic Animals" Marine Drugs 12, no. 5: 2623-2632. https://doi.org/10.3390/md12052623