Antimicrobial Activity of the Marine Alkaloids, Clathrodin and Oroidin, and Their Synthetic Analogues
Abstract
:1. Introduction

2. Results and Discussion
2.1. Design

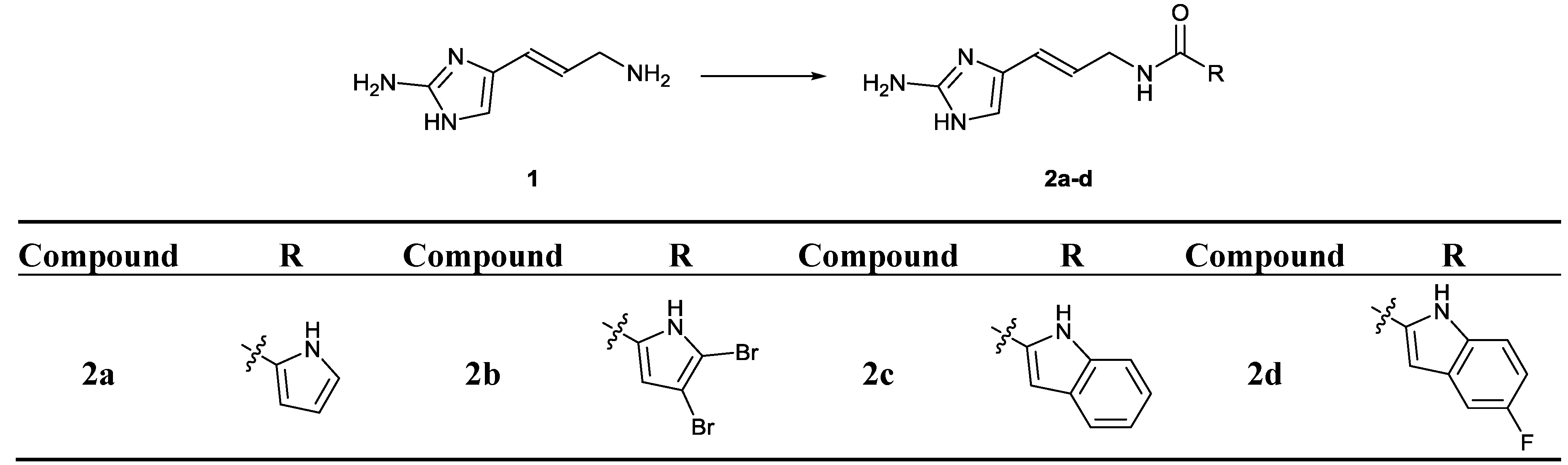
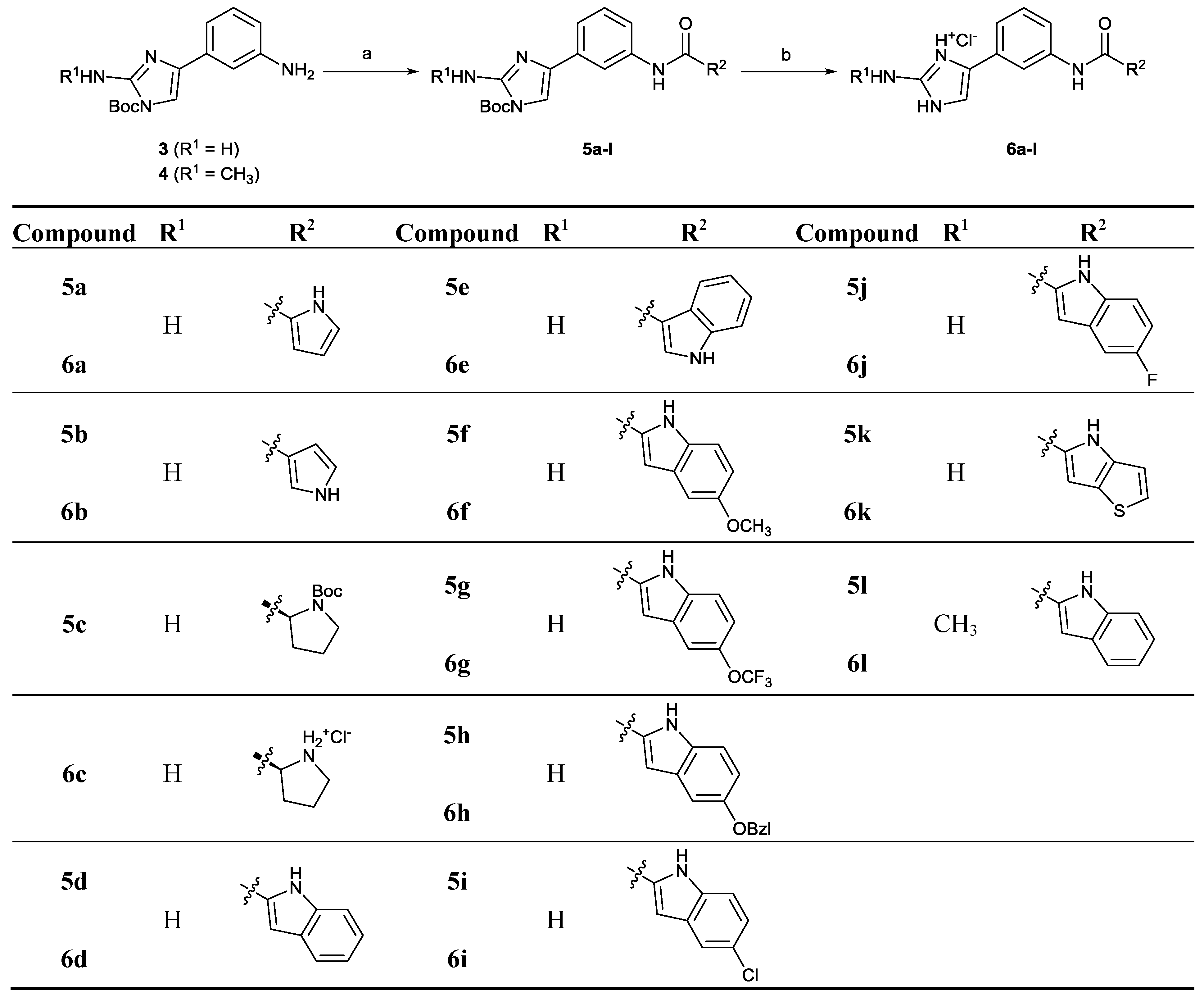
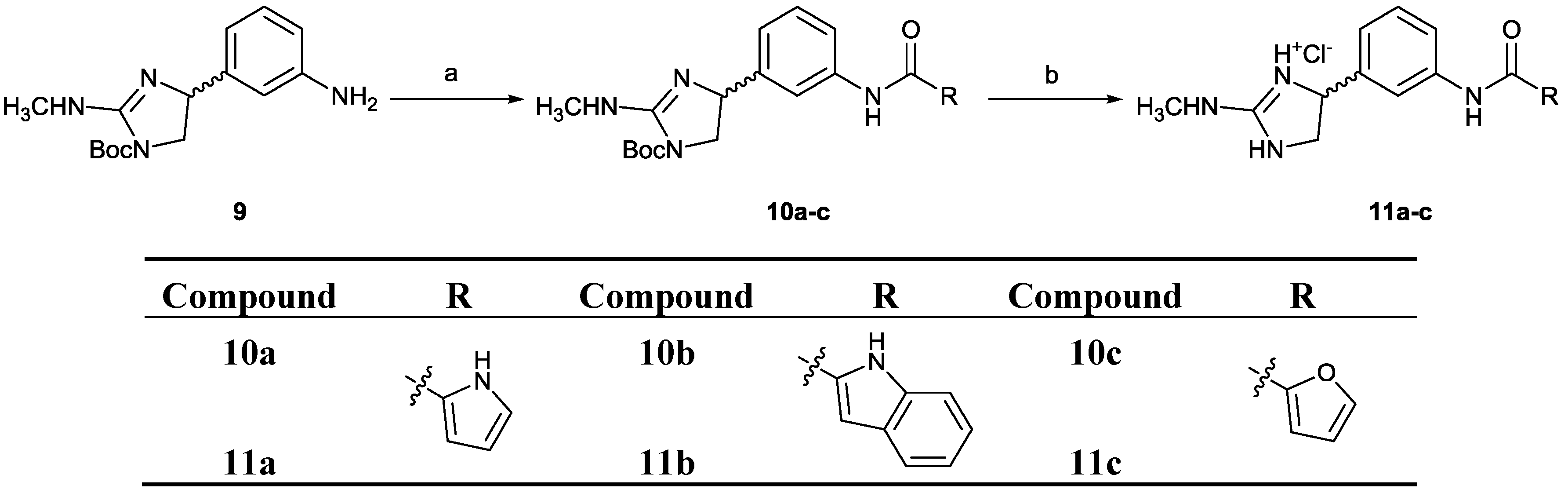

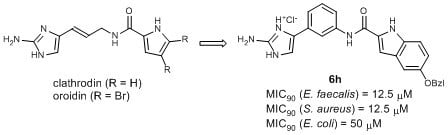
2.2. Chemistry
2.3. Biological Evaluation

| Cpd | Enterococcus faecalis (ATCC 29212) | Staphylococcus aureus (ATCC 25923) | Escherichia coli (ATCC 25922) | Candida albicans (ATCC 90028) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC90 (µM) | MIC50 (µM) | SI | MIC90 (µM) | MIC50 (µM) | SI | MIC90 (µM) | MIC50 (µM) | SI | MIC90 (µM) | MIC50 (µM) | SI | |
| 2b | 100 | 30.6 | >3.3 | |||||||||
| 5i | 75 | 11.7 | >8.6 | |||||||||
| 6d | 50 | 34.0 | 1.2 | 50 | 29.4 | 1.4 | ||||||
| 6f | 50 | 11.9 | 2.7 | |||||||||
| 6g | 25 | 11.4 | 2.7 | 25 | 9.0 | 3.5 | 25 | 17.7 | 1.8 | 50 | 30.5 | 1.0 |
| 6h | 12.5 | 8.0 | 2.7 | 12.5 | 7.3 | 2.9 | 50 | 30.4 | 0.7 | |||
| 6i | 50 | 7.2 | 3.0 | |||||||||
| 6j | 100 | 27.3 | 0.7 | 50 | 22.1 | 0.9 | ||||||
| 6k | 100 | 27.1 | 2.0 | |||||||||
| 6l | 100 | 54.2 | 1.0 | 75 | 10.8 | 4.9 | ||||||
| 7 | 100 | 11.6 | 8.1 | |||||||||
| 8 | 100 | 40.5 | >2.5 | |||||||||
| Compound | Cytotoxicity |
|---|---|
| IC50 (µM) | |
| 2b | >100 * |
| 5i | >100 * |
| 6d | 42.3 |
| 6f | 32.2 |
| 6g | 31.0 |
| 6h | 21.2 |
| 6i | 21.7 |
| 6j | 19.8 |
| 6k | 53.6 |
| 6l | 52.3 |
| 7 | 94.0 |
| 8 | >100 * |
3. Experimental Section
3.1. Determination of Antimicrobial Activity
3.1.1. Microbial Strains
3.1.2. Microdilution Assay
3.2. Determination of Mammalian Cell Cytotoxicity
3.2.1. Cell Culture
3.2.2. ATP Assay
3.3. Chemistry: General
3.4. Synthetic Procedures
3.4.1. General Procedure A: Synthesis of compounds 2a–d
3.4.2. General Procedure B: Synthesis of Compounds 5a–l and 10a–c (with 5f as an Example)
3.4.3. tert-Butyl 2-Amino-4-(3-(5-hydroxy-1H-indole-2-carboxamido)phenyl)-1H-imidazole-1-carboxylate (7)
3.4.4. General Procedure C: Synthesis of Compounds 6a–l, 8 and 11a–c (with 6f as an Example)
3.4.5. 1-Benzyl-4-(3-nitrophenyl)-1H-imidazol-2-amine (13)
3.4.6. 4-(3-Aminophenyl)-1-benzyl-1H-imidazol-2-amine (14)
3.4.7. N-(3-(2-Amino-1-benzyl-1H-imidazol-4-yl)phenyl)-1H-pyrrole-2-carboxamide (15)
3.4.8. 4-(3-(((1H-Pyrrol-2-yl)methyl)amino)phenyl)-1-benzyl-1H-imidazol-2-amine (16)
4. Conclusions
Abbreviations
| ATCC | American Type Culture Collection |
| d | doublet |
| dd | doublet of doublets |
| ddd | doublet of doublet of doublets |
| DMF | N,N-dimethylformamide |
| DMSO | dimethylsulfoxide |
| dt | doublet of triplets |
| ESI | electrospray ionization |
| EtOH | ethanol |
| FT-IR | fourier transform infrared |
| HRMS | high resolution mass spectrometry |
| Huh-7 | human hepatocellular carcinoma cell line |
| IR | infrared |
| MOPS | 3-(N-morpholino)propanesulfonic acid |
| mp | melting point |
| NMM | N-methylmorpholine |
| R | radical |
| RPMI-1640 | Roswell Park Memorial Institute medium 1640 |
| rt | room temperature |
| s | singlet |
| TBTU | N,N,N′,N′-tetramethyl-O-(benzotriazol-1-yl)uronium tetrafluoroborate |
| td | triplet of doublets |
| THF | tetrahydrofuran |
| TLC | thin layer chromatography |
| TMS | tetramethylsilane |
| Vis | visible light |
Supplementary Files
Acknowledgments
Conflicts of Interest
References
- Al-Sabi, A.; McArthur, J.; Ostroumov, V.; French, R.J. Marine toxins that target voltage-gated sodium channels. Mar. Drugs 2006, 4, 157–192. [Google Scholar] [CrossRef]
- Doshi, G.M.; Aggarwal, G.V.; Martins, E.A.; Shanbhag, P.P. Novel antibiotics from marine sources. Int. J. Pharm. Sci. Nanotech. 2011, 4, 1446–1461. [Google Scholar]
- Al-Mourabit, A.; Zancanella, M.A.; Tilvi, S.; Romo, D. Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids. Nat. Prod. Rep. 2011, 28, 1229–1260. [Google Scholar] [CrossRef]
- Forte, B.; Malgesini, B.; Piutti, C.; Quartieri, F.; Scolaro, A.; Papeo, G. A submarine journey: The pyrrole-imidazole alkaloids. Mar. Drugs 2009, 7, 705–753. [Google Scholar] [CrossRef]
- Al Mourabit, A.; Potier, P. Sponge’s molecular diversity through the ambivalent reactivity of 2-aminoimidazole: A universal chemical pathway to the oroidin-based pyrrole-imidazole alkaloids and their palau’amine congeners. Eur. J. Org. Chem. 2001, 237–243. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Chopra, I.; Schofield, C.; Everett, M.; O’Neill, A.; Miller, K.; Wilcox, M.; Frere, J.M.; Dawson, M.; Czapiewski, L.; Urleb, U.; et al. Treatment of health-care-associated infections caused by gram-negative bacteria: A consensus statement. Lancet Infect. Dis. 2008, 8, 133–139. [Google Scholar] [CrossRef]
- Theuretzbacher, U. Accelerating resistance, inadequate antibacterial drug pipelines and international responses. Int. J. Antimicrob. Agents 2012, 39, 295–299. [Google Scholar] [CrossRef]
- Walsh, T.R.; Toleman, M.A. The emergence of pan-resistant gram-negative pathogens merits a rapid global political response. J. Antimicrob. Chemoth. 2012, 67, 1–3. [Google Scholar] [CrossRef]
- Thompson, R.J.; Bobay, B.G.; Stowe, S.D.; Olson, A.L.; Peng, L.L.; Su, Z.M.; Actis, L.A.; Melander, C.; Cavanagh, J. Identification of BfmR, a response regulator involved in biofilm development, as a target for a 2-aminoimidazole-based antibiofilm agent. Biochemistry 2012, 51, 9776–9778. [Google Scholar] [CrossRef]
- Steenackers, H.P.L.; Ermolat’ev, D.S.; Savaliya, B.; De Weerdt, A.; De Coster, D.; Shah, A.; Van der Eycken, E.V.; De Vos, D.E.; Vanderleyden, J.; De Keersmaecker, S.C.J. Structure-activity relationship of 2-hydroxy-2-aryl-2,3-dihydro-imidazo[1,2-a]pyrimidinium salts and 2N-substituted 4(5)-aryl-2-amino-1H-imidazoles as inhibitors of biofilm formation by Salmonella typhimurium and Pseudomonas aeruginosa. Bioorg. Med. Chem. 2011, 19, 3462–3473. [Google Scholar] [CrossRef]
- Steenackers, H.P.L.; Ermolat’ev, D.S.; Savaliya, B.; De Weerdt, A.; De Coster, D.; Shah, A.; Van der Eycken, E.V.; De Vos, D.E.; Vanderleyden, J.; De Keersmaecker, S.C.J. Structure-activity relationship of 4(5)-aryl-2-amino-1H-imidazoles, N1-substituted 2-aminoimidazoles and imidazo[1,2-a]pyrimidinium salts as inhibitors of biofilm formation by Salmonella typhimurium and Pseudomonas aeruginosa. J. Med. Chem. 2011, 54, 472–484. [Google Scholar] [CrossRef]
- Rogers, S.A.; Huigens, R.W.; Cavanagh, J.; Melander, C. Synergistic effects between conventional antibiotics and 2-aminoimidazole-derived antibiofilm agents. Antimicrob. Agents Chemother. 2010, 54, 2112–2118. [Google Scholar] [CrossRef]
- Rogers, S.A.; Huigens, R.W.; Melander, C. A 2-aminobenzimidazole that inhibits and disperses gram-positive biofilms through a zinc-dependent mechanism. J. Am. Chem. Soc. 2009, 131, 9868–9869. [Google Scholar] [CrossRef]
- Richards, J.J.; Reyes, S.; Stowe, S.D.; Tucker, A.T.; Ballard, T.E.; Mathies, L.D.; Cavanagh, J.; Melander, C. Amide isosteres of oroidin: Assessment of antibiofilm activity and C. elegans toxicity. J. Med. Chem. 2009, 52, 4582–4585. [Google Scholar] [CrossRef]
- Richards, J.J.; Reed, C.S.; Melander, C. Effects of N-pyrrole substitution on the anti-biofilm activities of oroidin derivatives against Acinetobacter baumannii. Bioorg. Med. Chem. Lett. 2008, 18, 4325–4327. [Google Scholar] [CrossRef]
- Bunders, C.A.; Richards, J.J.; Melander, C. Identification of aryl 2-aminoimidazoles as biofilm inhibitors in gram-negative bacteria. Bioorg. Med. Chem. Lett. 2010, 20, 3797–3800. [Google Scholar] [CrossRef]
- Ballard, T.E.; Richards, J.J.; Aquino, A.; Reed, C.S.; Melander, C. Antibiofilm activity of a diverse oroidin library generated through reductive acylation. J. Org. Chem. 2009, 74, 1755–1758. [Google Scholar] [CrossRef]
- Stowe, S.D.; Richards, J.J.; Tucker, A.T.; Thompson, R.; Melander, C.; Cavanagh, J. Anti-biofilm compounds derived from marine sponges. Mar. Drugs 2011, 9, 2010–2035. [Google Scholar] [CrossRef]
- Huigens, R.W.; Reyes, S.; Reed, C.S.; Bunders, C.; Rogers, S.A.; Steinhauer, A.T.; Melander, C. The chemical synthesis and antibiotic activity of a diverse library of 2-aminobenzimidazole small molecules against MRSA and multidrug-resistant A. baumannii. Bioorg. Med. Chem. 2010, 18, 663–674. [Google Scholar] [CrossRef]
- Harris, T.L.; Worthington, R.J.; Melander, C. A facile synthesis of 1,5-disubstituted-2-amino imidazoles: Antibiotic activity of a first generation library. Bioorg. Med. Chem. Lett. 2011, 21, 4516–4519. [Google Scholar] [CrossRef]
- Rogers, S.A.; Lindsey, E.A.; Whitehead, D.C.; Mullikin, T.; Melander, C. Synthesis and biological evaluation of 2-aminoimidazole/carbamate hybrid anti-biofilm and anti-microbial agents. Bioorg. Med. Chem. Lett. 2011, 21, 1257–1260. [Google Scholar] [CrossRef]
- Hammami, S.; Bergaoui, A.; Boughalleb, N.; Romdhane, A.; Khoja, I.; Kamel, M.B.; Mighri, Z. Antifungal effects of secondary metabolites isolated from marine organisms collected from the Tunisian coast. C. R. Chim 2010, 13, 1397–1400. [Google Scholar] [CrossRef]
- Scala, F.; Fattorusso, E.; Menna, M.; Taglialatela-Scafati, O.; Tierney, M.; Kaiser, M.; Tasdemir, D. Bromopyrrole alkaloids as lead compounds against protozoan parasites. Mar. Drugs 2010, 8, 2162–2174. [Google Scholar] [CrossRef]
- Tasdemir, D.; Topaloglu, B.; Perozzo, R.; Brun, R.; O’Neill, R.; Carballeira, N.M.; Zhang, X.J.; Tonge, P.J.; Linden, A.; Ruedi, P. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg. Med. Chem. 2007, 15, 6834–6845. [Google Scholar] [CrossRef]
- Bernan, V.S.; Roll, D.M.; Ireland, C.M.; Greenstein, M.; Maiese, W.M.; Steinberg, D.A. A study on the mechanism of action of sceptrin, an antimicrobial agent isolated from the south-pacific sponge Agelas mauritiana. J. Antimicrob. Chemother. 1993, 32, 539–550. [Google Scholar] [CrossRef]
- Little, T.L.; Webber, S.E. A simple and practical synthesis of 2-aminoimidazoles. J. Org. Chem. 1994, 59, 7299–7305. [Google Scholar] [CrossRef]
- Zidar, N.; Jakopi, Ž.; Madge, D.J.; Chan, F.; Tytgat, J.; Peigneur, S.; Sollner-Dolenc, M.; Tomasić, T.; Ilaš, J.; Peterlin Mašič, L.; et al. Substituted 4-phenly-2-aminoimidazoles and 4-phenyl-4,5-dihydro-2-aminoimidazoles as volzage-gated sodium channel modulators. Eur. J. Med. Chem. 2014, 74, 23–30. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; CLSI: Wayne, PA, USA, 2013; pp. 1–206. [Google Scholar]
- Vicente, E.; Perez-Silanes, S.; Lima, L.M.; Ancizu, S.; Burguete, A.; Solano, B.; Villar, R.; Aldana, I.; Monge, A. Selective activity against Mycobacterium tuberculosis of new quinoxaline 1,4-di-N-oxides. Bioorg. Med. Chem. 2009, 17, 385–389. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zidar, N.; Montalvão, S.; Hodnik, Ž.; Nawrot, D.A.; Žula, A.; Ilaš, J.; Kikelj, D.; Tammela, P.; Mašič, L.P. Antimicrobial Activity of the Marine Alkaloids, Clathrodin and Oroidin, and Their Synthetic Analogues. Mar. Drugs 2014, 12, 940-963. https://doi.org/10.3390/md12020940
Zidar N, Montalvão S, Hodnik Ž, Nawrot DA, Žula A, Ilaš J, Kikelj D, Tammela P, Mašič LP. Antimicrobial Activity of the Marine Alkaloids, Clathrodin and Oroidin, and Their Synthetic Analogues. Marine Drugs. 2014; 12(2):940-963. https://doi.org/10.3390/md12020940
Chicago/Turabian StyleZidar, Nace, Sofia Montalvão, Žiga Hodnik, Dorota A. Nawrot, Aleš Žula, Janez Ilaš, Danijel Kikelj, Päivi Tammela, and Lucija Peterlin Mašič. 2014. "Antimicrobial Activity of the Marine Alkaloids, Clathrodin and Oroidin, and Their Synthetic Analogues" Marine Drugs 12, no. 2: 940-963. https://doi.org/10.3390/md12020940
APA StyleZidar, N., Montalvão, S., Hodnik, Ž., Nawrot, D. A., Žula, A., Ilaš, J., Kikelj, D., Tammela, P., & Mašič, L. P. (2014). Antimicrobial Activity of the Marine Alkaloids, Clathrodin and Oroidin, and Their Synthetic Analogues. Marine Drugs, 12(2), 940-963. https://doi.org/10.3390/md12020940