Polyoxygenated Steroids from the Octocoral Leptogorgia punicea and in Vitro Evaluation of Their Cytotoxic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 31.8 | 32.0 | 32.3 | 32.2 | 32.4 |
| 2 | 30.5 | 30.7 | 30.9 | 30.9 | 31.0 |
| 3 | 67.2 | 67.3 | 67.6 | 67.6 | 67.6 |
| 4 | 40.4 | 40.5 | 40.8 | 40.8 | 40.9 |
| 5 | 75.9 | 75.3 | 76.3 | 76.3 | 75.7 |
| 6 | 75.7 | 75.9 | 76.0 | 76.1 | 76.3 |
| 7 | 32.2 | 31.6 | 32.6 | 32.6 | 32.0 |
| 8 | 27.1 | 30.9 | 27.5 | 27.6 | 31.3 |
| 9 | 48.0 | 45.5 | 48.5 | 48.5 | 45.9 |
| 10 | 39.0 | 38.5 | 39.4 | 39.4 | 38.9 |
| 11 | 69.0 | 21.3 | 68.3 | 69.4 | 21.5 |
| 12 | 49.4 | 34.7 | 49.6 | 49.6 | 34.5 |
| 13 | 41.7 | 40.5 | 42.0 | 42.0 | 47.1 |
| 14 | 57.4 | 55.4 | 57.9 | 57.9 | 55.8 |
| 15 | 23.8 | 23.9 | 28.6 | 28.4 | 24.3 |
| 16 | 24.1 | 28.1 | 24.4 | 24.4 | 27.6 |
| 17 | 56.8 | 56.4 | 56.9 | 57.0 | 56.6 |
| 18 | 14.8 | 61.2 | 15.4 | 15.4 | 60.7 |
| 19 | 19.6 | 16.5 | 20.0 | 20.0 | 16.9 |
| 20 | 35.8 | 36.3 | 40.5 | 40.3 | 40.3 |
| 21 | 18.7 | 19.3 | 21.3 | 21.2 | 22.7 |
| 22 | 36.1 | 36.3 | 138.1 | 133.7 | 137.8 |
| 23 | 27.8 | 23.6 | 126.0 | 135.5 | 127.8 |
| 24 | 39.5 | 39.5 | 42.3 | - | 42.3 |
| 25 | 28.0 | 28.0 | 28.9 | 31.3 | 28.9 |
| 26 | 22.5 | 22.6 | 22.7 | 23.2 | 22.7 |
| 27 | 22.8 | 22.8 | 22.7 | 23.2 | 22.7 |
| 6-OAc | 170.3 | 171.3 | 170.7 | 170.7 | 170.5 |
| 21.5 | 21.4 | 21.9 | 21.8 | 21.8 |
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 1.65 (m a) | 1.45 (m a) | 1.65 (m a) | 1.65 (m a) | 1.45 (m a) |
| 2α | 1.87 (m a) | 1.86 (m a) | 1.87 (m a) | 1. 87 (m a) | 1.86 (m a) |
| 2β | 1.58 (m a) | 1.52 (m a) | 1.57 (m a) | 1.58 (m a) | 1.54 (m a) |
| 3 | 4.05 (m a) | 4.09 (m a) | 4.05 (m a) | 4.06 (m a) | 4.08 (m a) |
| 4α | 1.54 (m a) | 1.58 (m a) | 1.54(m a) | 1.56(m a) | 1.57 (m a) |
| 4β | 1.95 (m a) | 1. 85 (m a) | 1.95 (m a) | 1.96 (m a) | 1.85 (m a) |
| 6 | 4.67 (dd, 3.2,3.2) | 4.7 (dd, 3.0,3.0) | 4.66 (dd, 3.0,3.0) | 4.66 (dd, 3.1,3.1) | 4.70 (dd, 2.8,2.8) |
| 7 | 1.70 (m a) | 1.61 (m a) | 1.70 (m a) | 1.70 (m a) | 1.59 (m a) |
| 8 | 1.96 (m a) | 1.68 (m a) | 1.95 (m a) | 1.96 (m a) | 1.67 (m a) |
| 9 | 1.51 (m a) | 1.38 (m a) | 1.50 (m a) | 1.51 (m a) | 1.38 (m a) |
| 11α | 4.19 (dd, 6.5, 3.2) | 1.48 (m a) | 4.19 (dd, 6.5, 3.2) | 4.19 (dd, 6.3, 3.3) | 1.48 (m a) |
| 11β | - | 1.31 (m a) | - | - | 1.30 (m a) |
| 12α | 1.45 (m a) | 1.04 (m a) | 1.44 (m a) | 1.46 (m a) | 1.00 (m a) |
| 12β | 2.15 (dd, 14.0, 2.7) | 2,46 (td, 12.7, 3.0) | 2.11 (dd, 14.0, 2.7) | 2.11 (dd, 14.2, 2.9) | 2.42 (td, 12.7, 3.0) |
| 14 | 1.08 (m a) | 1.24 (m a) | 1.08 (m a) | 1.07 (m a) | 1.21 (m a) |
| 15α | 1.32 (m a) | 1.57 (m a) | 1.66 (m a) | 1.64 (m a) | 1.55 (m a) |
| 15β | 1.10 (m a) | 1.01 (m a) | 1.26 (m a) | 1.25 (m a) | 0.95 (m a) |
| 16α | 1.59 (m a) | 1.89 (m a) | 1.55(m a) | 1.57 (m a) | 1.75 (m a) |
| 16β | 1.08 (m a) | 1.41 (m a) | 1.08 (m a) | 1.08 (m a) | 1.42 (m a) |
| 17 | 1.06 (m a) | 1.20 (m a) | 1.10 (m a) | 1.08 (m a) | 1.28 (m a) |
| 18a | 0.91 (s) | 3.73 (dd, 11.4, 4.7) | 0.93 (s) | 0.92 (s) | 3.62 (d, 11.7) |
| 18b | 3.61 (dd, 11.4, 4.7) | 3.55 (d, 11.7) | |||
| 19 | 1.38 (s) | 1.15 (s) | 1.38 (s) | 1.38 (s) | 1.15 (s) |
| 20 | 1.36 (m a) | 1.57 (m a) | 2.01 (m a) | 1.97 (m a) | 2.29 (dd, 15.3, 8.4) |
| 21 | 0.92 (d, 6.6) | 1.03 (d, 6.6) | 1.01 (d, 6.5) | 1.00 (d, 6.6) | 1.09 (d, 6.6) |
| 22 | 1.32 (m a) 0.98 (m a) | 1.37 (m a) 1.04 (m a) | 5.16 (dd, 15.0, 9.0) | 5.13 (dd, 15.0, 8.4) | 5.33 (dd, 15.0, 8.5) |
| 23 | 1.81 (m a) 1.25 (m a) | 1.18 (m a) 1,33 (m a) | 5.26 (dd, 15.0, 7.0) | 5.26 (dd, 15.0, 6.6) | 5.37 (dd, 15.0, 6.5) |
| 24 | 1.11 (m a) | 1.13 (m a) | 1.82 (m a) | - | 1.85 (m a) |
| 25 | 1.51 (m a) | 1.51 (m a) | 1.56 (m a) | 2.18 (m a) | 1.58 (m a) |
| 26 | 0.86 (d, 6.6) | 0.86 (d, 6.6) | 0.86 (d, 6.7) | 0.94 (d, 6.6) | 0.87 (d, 6.7) |
| 27 | 0.87 (d, 6.6) | 0.87 (d, 6.6) | 0.86(d, 6.7) | 0.94 (d, 6.6) | 0.87 (d, 6.7) |
| 6-OAc | 2.07 (s) | 2.06 (s) | 2.07(s) | 2.07 (s) | 2.06 (s) |
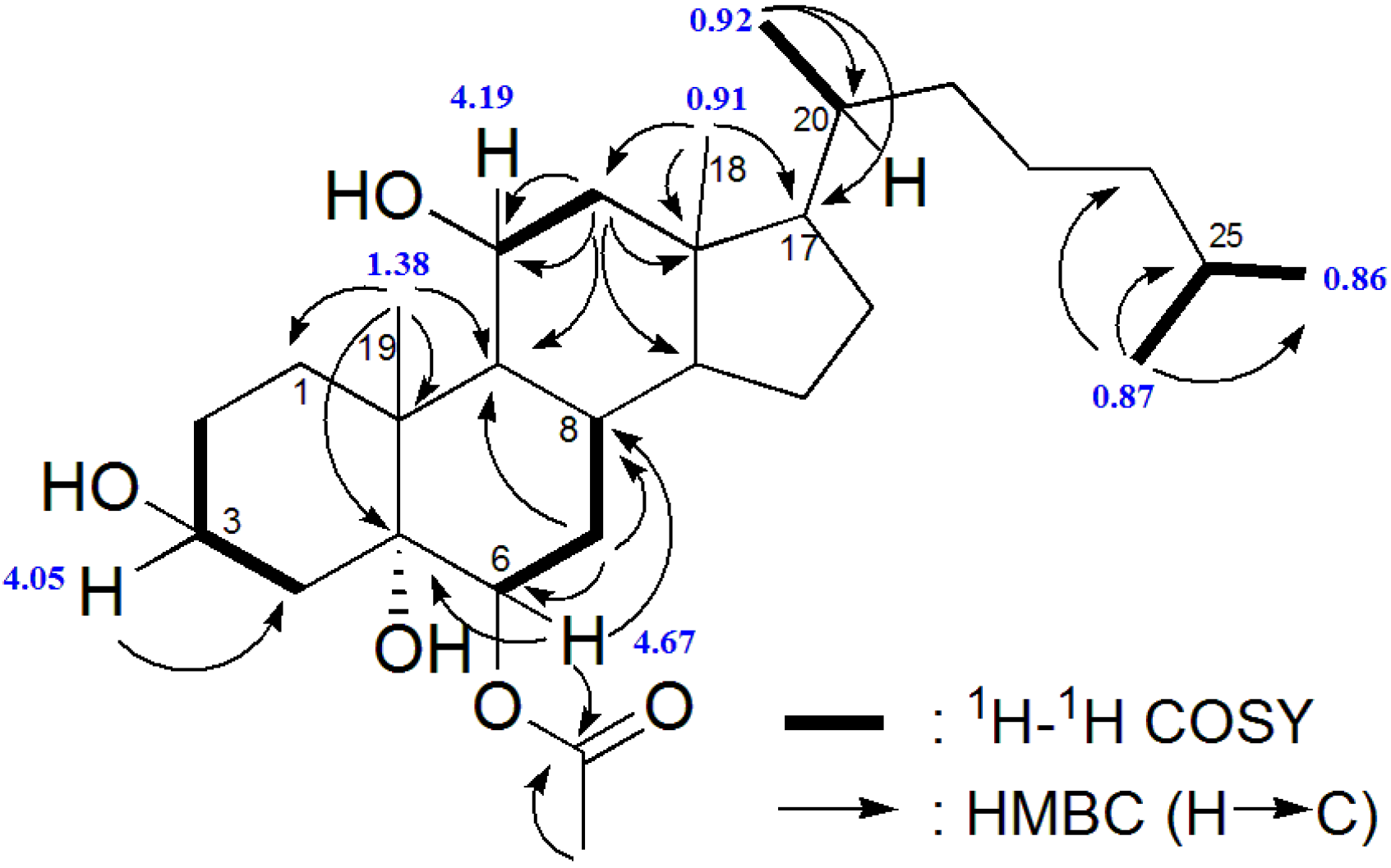
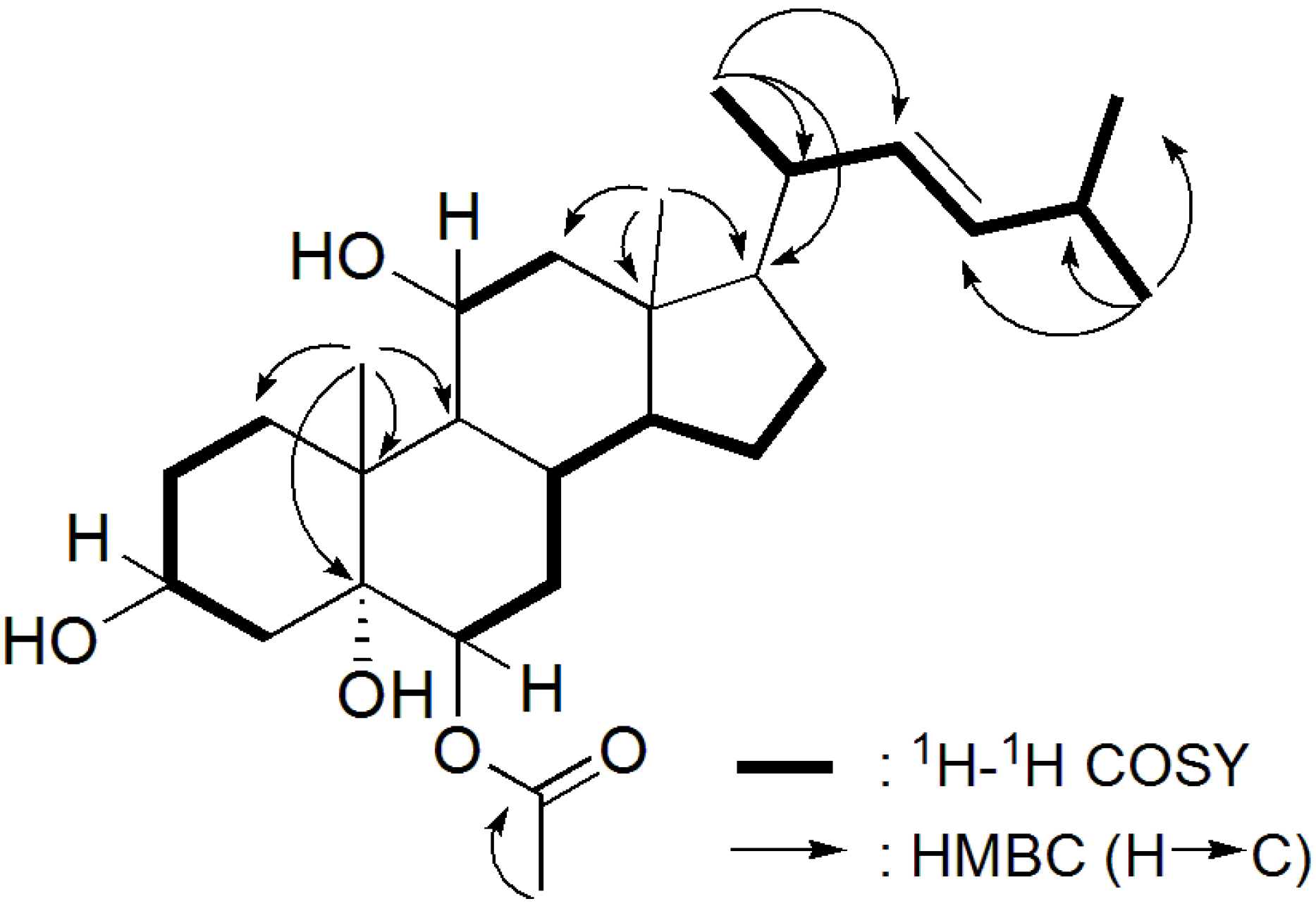
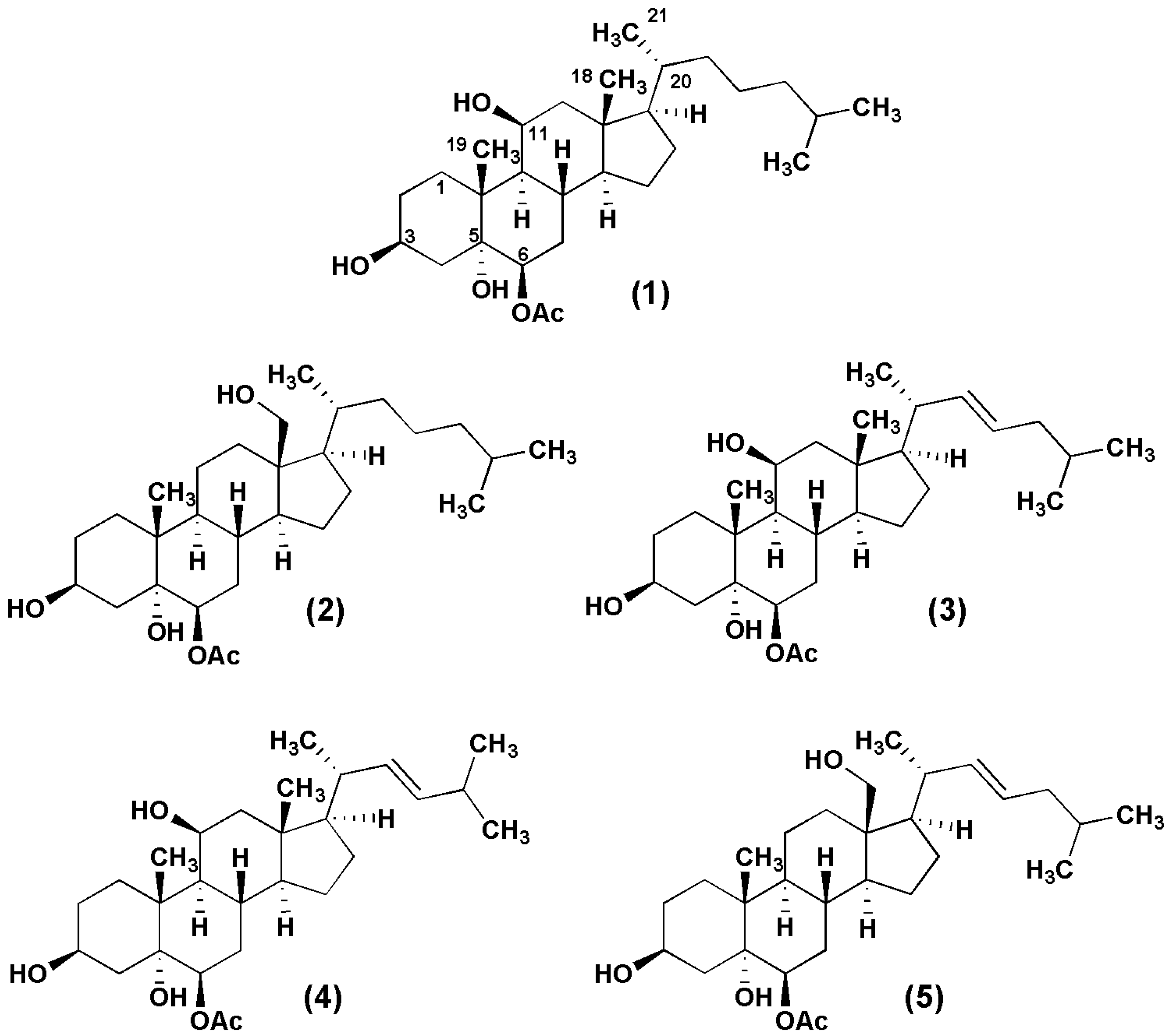
2.2. Biological Activity
| Punicinol | IC50 24 h (µM) | IC50 48 h (µM) |
|---|---|---|
| A | 11.8 ± 1.4 | 9.6 ± 1.1 |
| B | 13.6 ± 2.1 | 9.7 ± 1.7 |
| C | 47.9 ± 1.5 | 35.9 ± 1.4 |
| D | 81.3 ± 5.0 | 73.3 ± 0.6 |
| E | 59.0 ± 2.1 | 35.8 ± 1.0 |
| Cisplatin | 24.8 ± 2.1 | 16.2 ± 2.4 |
| Paclitaxel | >1.0 | 0.247 ± 0.04 |
| Compounds Combination Ratio | Punicinol A (µM) | Paclitaxel (µM) | Experimental CI Values | Description (Graded Symbols) |
|---|---|---|---|---|
| 4 × IC50 | 40 | 1.000 | 1.218 | Moderate antagonism (++) |
| 2 × IC50 | 20 | 0.500 | 1.248 | Moderate antagonism (++) |
| 1 × IC50 | 10 | 0.250 | 0.519 | Synergism (+++) |
| 0.5 × IC50 | 5 | 0.125 | 0.485 | Synergism (+++) |
| 0.25 × IC50 | 2.5 | 0.063 | 0.346 | Synergism (+++) |
| 0.125 × IC50 | 1.25 | 0.031 | 0.180 | Strong synergism (++++) |
| 0.062 × IC50 | 0.625 | 0.016 | 0.116 | Strong synergism (++++) |
| 0.031 × IC50 | 0.31 | 0.008 | 0.095 | Very Strong synergism (+++++) |
| Compounds Combination ratio | Punicinol B (µM) | Paclitaxel (µM) | Experimental CI values | Description (graded symbols) |
|---|---|---|---|---|
| 4 × IC50 | 40 | 1.000 | 0.735 | Moderate Synergism (++) |
| 2 × IC50 | 20 | 0.500 | 0.633 | Synergism (+++) |
| 1× IC50 | 10 | 0.250 | 0.922 | Additive effect (±) |
| 0.5 × IC50 | 5 | 0.125 | 1.024 | Additive effect (±) |
| 0.25 × IC50 | 2.5 | 0.063 | 6.381 | Strong antagonism (++++) |
| 0.125 × IC50 | 1.25 | 0.031 | 3.191 | Antagonism (−−−) |
| 0.062 × IC50 | 0.625 | 0.016 | 1.595 | Antagonism (−−−) |
| 0.031 × IC50 | 0.31 | 0.008 | 0.799 | Synergism (+++) |
3. Experimental Section
3.1. General
3.2. Biological Material Collection
3.3. Extraction and Isolation
3.4. Biological Activity
3.4.1. Cell Culture
3.4.2. Sulforhodamine B Assay
3.4.3. Synergistic Effects of Punicinols A and B in Combination with Paclitaxel
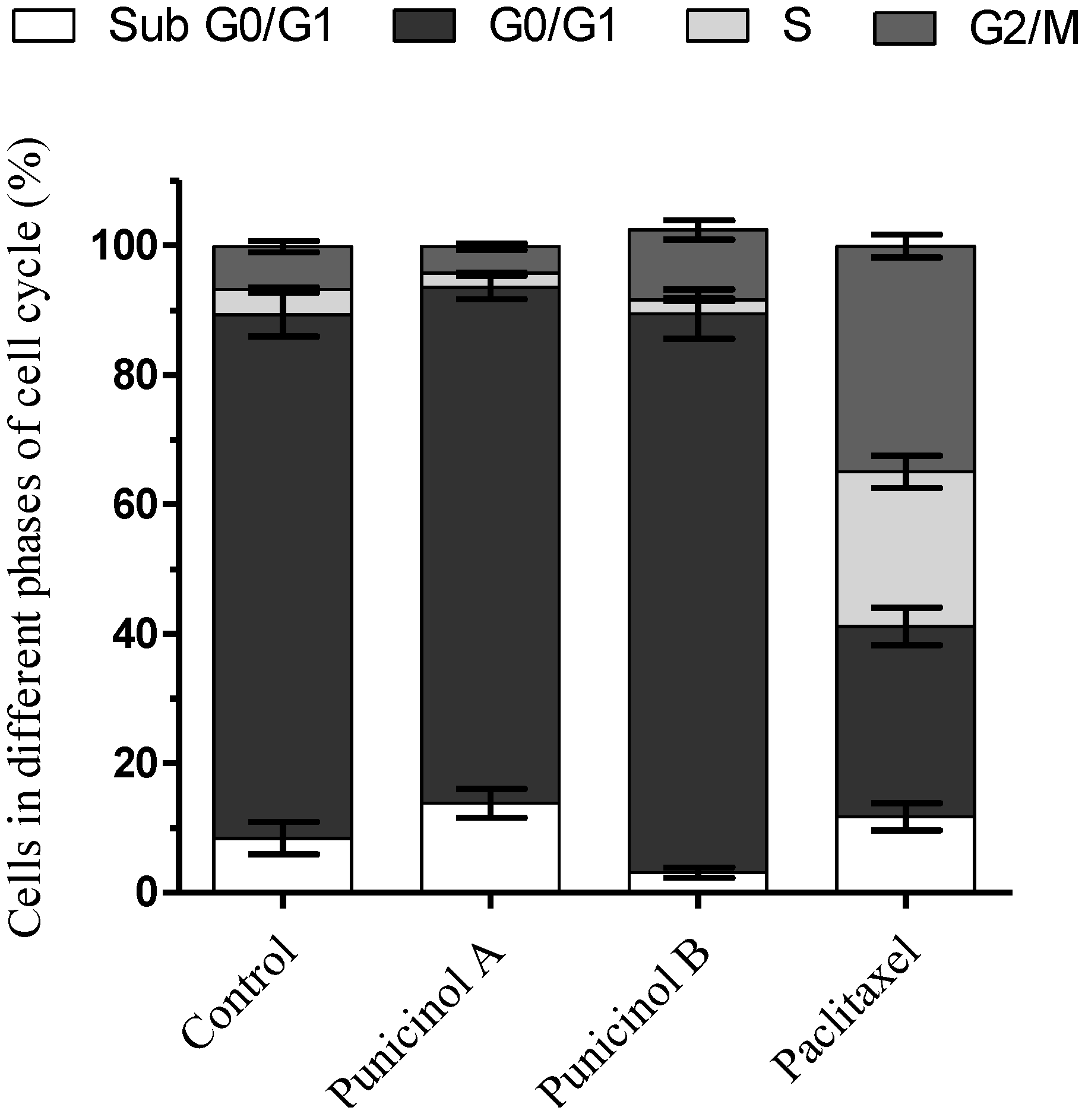
3.4.4. Cell Cycle Analysis by Flow Cytometry
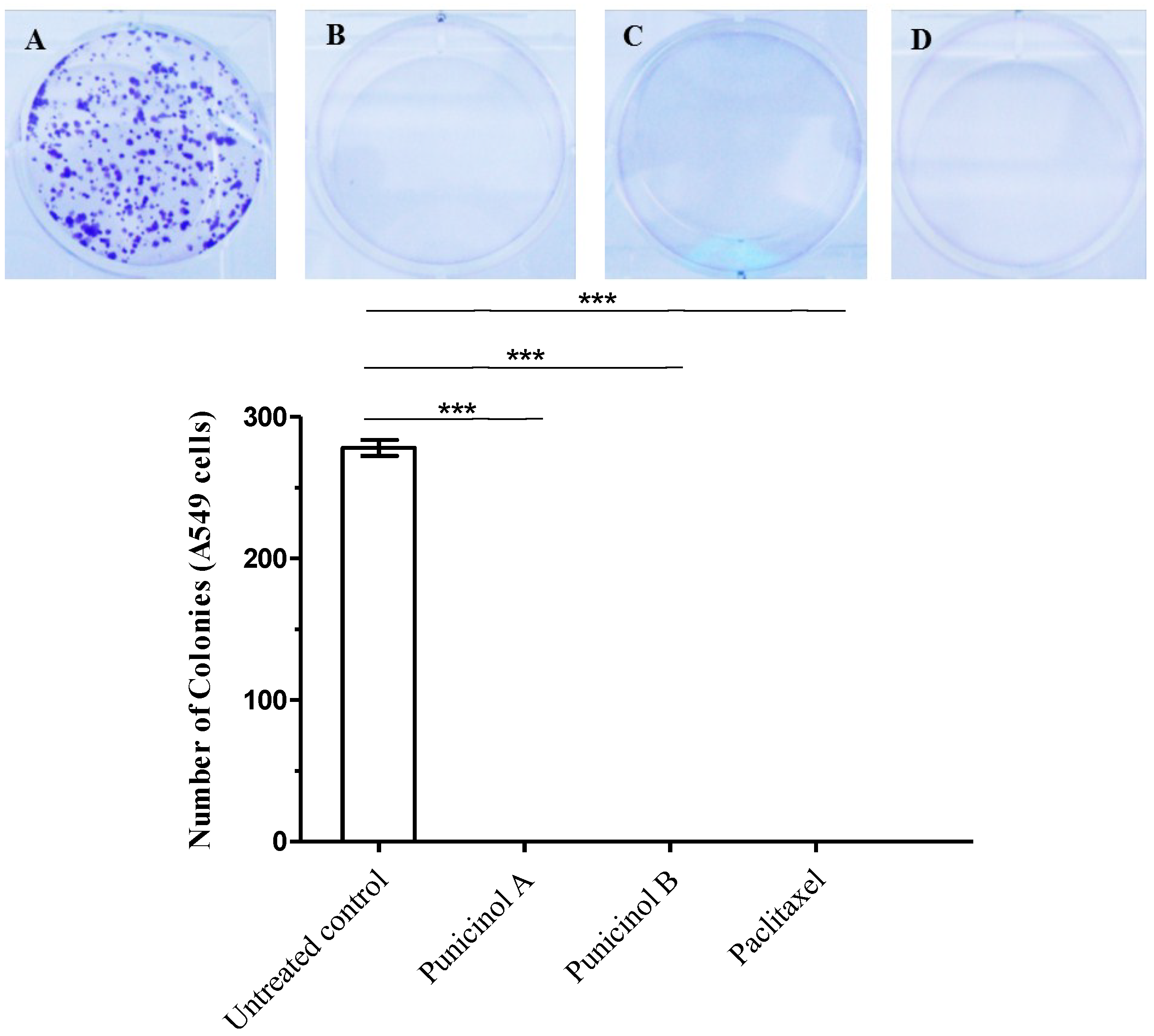
3.4.5. Clonogenic Assay
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.D. Primeiro registro de Leptogorgia punicea (Milne-Edwards & Haime) (Cnidaria, Octocorallia) para o estado do Maranhão, Brasil. Rev. Bras. Zool. 2005, 22, 810–811. [Google Scholar] [CrossRef]
- Breedy, O.; Guzman, H.M. Leptogorgia ignita, a new shallow-water coral species (Octocorallia: Gorgoniidae) from the tropical eastern Pacific. J. Mar. Biol. Assoc. UK 2008, 88, 893–899. [Google Scholar] [CrossRef]
- Ksebati, M.B.; Ciereszko, L.S.; Schmitz, F.J. 11β, 12β-Epoxypukalide, a furanocembranolide from the gorgonian Leptogorgia setacea. J. Nat. Prod. 1984, 47, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.; Capson, T.L.; Guzmán, H.M.; González, J.; Ortega-Barría, E.; Quiñoá, E.; Riguera, R. Leptolide, a New Furanocembranolide Diterpene from Leptogorgia alba. J. Nat. Prod. 2005, 68, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Díaz-Marrero, A.R.; Brito, I.; Cueto, M.; D’Croz, L.; Darias, J. The oxidation profile at C-18 of furanocembranolides may provide a taxonomical marker for several genera of octocorals. Tetrahedron 2007, 63, 9057–9062. [Google Scholar] [CrossRef]
- Shapo, J.L.; Moeller, P.D.; Galloway, S.B. Antimicrobial activity in the common seawhip, Leptogorgia virgulata (Cnidaria: Gorgonaceae). Comp. Biochem. Physiol. 2007, 148, 65–73. [Google Scholar] [CrossRef]
- Ortega, M.J.; Zubía, E.; Sánchez, M.C.; Carballo, J.L. Cembrane Diterpenes from the Gorgonian Leptogorgia laxa. J. Nat. Prod. 2008, 71, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Epifanio, R.A.; Maia, L.F.; Fenical, W. Chemical Defenses of the Endemic Brazialian Gorgonian Lophogorgia violacea Pallas (Octocorallia, Gorgonacea). J. Braz. Chem. Soc. 2000, 11, 584–591. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; Porras, G.; Cueto, M.; Croz, L.D.; Lorenzo, M.; San-Martín, A.; Darias, J. Leptogorgolide, a biogenetically interesting 1,4-diketo-cembranoid that reinforces the oxidation profile of C-18 as taxonomical marker for octocorals. Tetrahedron 2009, 65, 6029–6033. [Google Scholar] [CrossRef]
- Keyzers, R.A.; Gray, C.A.; Schleyer, M.H.; Whibley, C.E.; Hendricks, D.T.; Davies-Coleman, M.T. Malonganenones A–C, novel tetraprenylated alkaloids from the Mozambique gorgonian Leptogorgia gilchristi. Tetrahedron 2006, 62, 2200–2206. [Google Scholar] [CrossRef]
- Cimino, G.; de Rosa, S.; de Stefano, S.; Sodano, G. 18-Hydroxy steroids from the Mediterranean gorgonian Leptogorgia sarmentosa. Experientia 1984, 40, 246–248. [Google Scholar] [CrossRef]
- Benvegnu, R.; Cimino, G.; de Rosa, S.; de Stefano, S. Guggulsterol-like steroids from the Mediterranean gorgonian Leptogorgia sarmentosa. Experientia 1982, 38, 1443–1444. [Google Scholar] [CrossRef]
- Epifanio, R.A.; Maia, L.F.; Pinto, A.C.; Hardt, I.; Fenical, W. Nature Products from the Gorgonian Lophogorgia punicea: Isolation and Structure Elucidation of an Unusual 17-hydroxy Sterol. J. Braz. C. Soc. 1998, 9, 187–192. [Google Scholar] [CrossRef]
- Garrido, L.; Zubia, E.; Ortega, M.J.; Salva, J. Isolation and structure elucidation of new cytotoxic steroids from the gorgonian Leptogorgia sarmentosa. Steroids 2000, 65, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.A. Marine polar steroids. Russ. Chem. Rev. 2001, 70, 673–715. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, B.S.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Carvalho, J.F.S.; Neves, M.A.C.; Silvestre, S.M.; Leitão, A.J.; Silva, M.M.C.; Melo, M.L.S. Anticancer steroids: Linking natural and semi-synthetic compounds. Nat. Prod. Rep. 2013, 30, 324–374. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Inoue, M.; Kamano, Y.; Herald, D.L.; Arm, C.; Dufresne, C.; Christie, N.D.; Schmidt, J.M.; Doubek, D.L.; Krup, T.S. Isolation and Structure of the Powerful Cell Growth Inhibitor Cephalostatin 1. J. Am. Chem. Soc. 1988, 110, 2006–2007. [Google Scholar] [CrossRef]
- Boonananwong, S.; Kongkathip, B.; Kongkathip, N. First synthesis of 3,16,20-polyoxygenated cholestanes, new cytotoxic steroids from the gorgonian Leptogorgia sarmentosa. Steroids 2008, 73, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Mellado, G.G.; Zubia, E.; Ortega, M.J.; López-González, P.J. New polyoxygenated steroids from the Antarctic octocoral Dasystenella acanthine. Steroids 2004, 69, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Bianco, E.M.; Oliveira, S.Q.; Rigotto, C.; Tonini, M.L.; Guimarães, T.R.; Bittencourt, F.; Gouvea, L.P.; Aresi, C.; Almeida, M.T.R.; Moritz, M.I.G.; et al. Anti-Infective potential of marine invertebrates and seaweeds from the Brazilian coast. Molecules 2013, 18, 5761–5778. [Google Scholar] [CrossRef] [PubMed]
- Kasal, A.; Budesinski, M.; Griffiths, W.J. Spectroscopic Methods of Steroid Analysis. In Steroid Analysis; Makin, H.L.J., Gower, D.B., Eds.; Springer: London, UK, 2010; p. 102. [Google Scholar]
- Yoshikawa, K.; Kanekuni, S.; Hanahusa, M.; Arihara, S.; Ohta, T. Polyhydroxylated Sterols from the Octocoral Dendronephthya gigantean. J. Nat. Prod. 2000, 63, 670–672. [Google Scholar] [CrossRef]
- Li, R.; Shao, C.; Qi, X.; Li, X.; Li, J.; Sun, L.; Wang, C. Polyoxygenated Sterols from the South China Sea Soft Coral Sinularia sp. Mar. Drugs 2012, 10, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, S.; Duh, C. Polyhydroxylated Steroids from Bamboo Coral Isis hippuris. Mar. Drugs 2011, 9, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Moldowan, J.M.; Tan, W.L.; Djerassi, C. 24ε-Methylcholestane-3β,5α,6β,25-Pentol 25-monoacetate, a novel polyoxygenated marine sterol. Steroids 1975, 26, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Iwamaru, A.; Iwado, E.; Kondo, S.; Newman, R.; Vera, B.; Rodríguez, A.; Kondo, Y. Eupalmerin acetate, a novel anticancer agent from Caribbean gorgonian octocorals, induces apoptosis in malignant glioma cells via the c-Jun NH2 terminal kinase pathway. Mol. Cancer Ther. 2007, 6, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tang, H.; Liu, B.S.; Li, T.J.; Sun, P.; Zhu, W.; Luo, Y.P.; Zhang, W. Tumor cell growth inhibitory activity and structure-activity relationship of polyoxygenated steroids from the gorgonian Menella kanisa. Steroids 2013, 78, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assays of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Silva, M.M.C.; Moreira, J.N.; Simões, S.; Sá e Melo, M.L. Selective cytotoxicity of oxysterols through structural modulation on rings a and b. synthesis, in vitro evaluation and SAR. J. Med. Chem. 2011, 54, 6375–6393. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Lu, X.; Tang, H.; Zhang, M.M.; Wang., P.; Sun, P.; Liu, Z.Y.; Wang, Z.L.; Li, L.; Rui, Y.C.; et al. 3β,5α,6β-Oxygenated sterols from the South China Sea gorgonian Muriceopsis flavida and their tumor cell growth inhibitory activity and apoptosis-inducing function. Steroids 2013, 78, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritz, M.I.G.; Marostica, L.L.; Bianco, É.M.; Almeida, M.T.R.; Carraro, J.L.; Cabrera, G.M.; Palermo, J.A.; Simões, C.M.O.; Schenkel, E.P. Polyoxygenated Steroids from the Octocoral Leptogorgia punicea and in Vitro Evaluation of Their Cytotoxic Activity. Mar. Drugs 2014, 12, 5864-5880. https://doi.org/10.3390/md12125864
Moritz MIG, Marostica LL, Bianco ÉM, Almeida MTR, Carraro JL, Cabrera GM, Palermo JA, Simões CMO, Schenkel EP. Polyoxygenated Steroids from the Octocoral Leptogorgia punicea and in Vitro Evaluation of Their Cytotoxic Activity. Marine Drugs. 2014; 12(12):5864-5880. https://doi.org/10.3390/md12125864
Chicago/Turabian StyleMoritz, Maria Izabel G., Lucas Lourenço Marostica, Éverson M. Bianco, Maria Tereza R. Almeida, João L. Carraro, Gabriela M. Cabrera, Jorge A. Palermo, Cláudia M. O. Simões, and Eloir P. Schenkel. 2014. "Polyoxygenated Steroids from the Octocoral Leptogorgia punicea and in Vitro Evaluation of Their Cytotoxic Activity" Marine Drugs 12, no. 12: 5864-5880. https://doi.org/10.3390/md12125864




