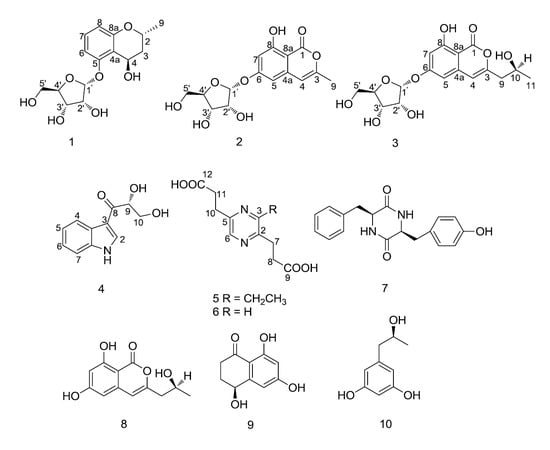
Five New Secondary Metabolites Produced by a Marine-Associated Fungus, Daldinia eschscholzii
Abstract
:1. Introduction
2. Results and Discussion
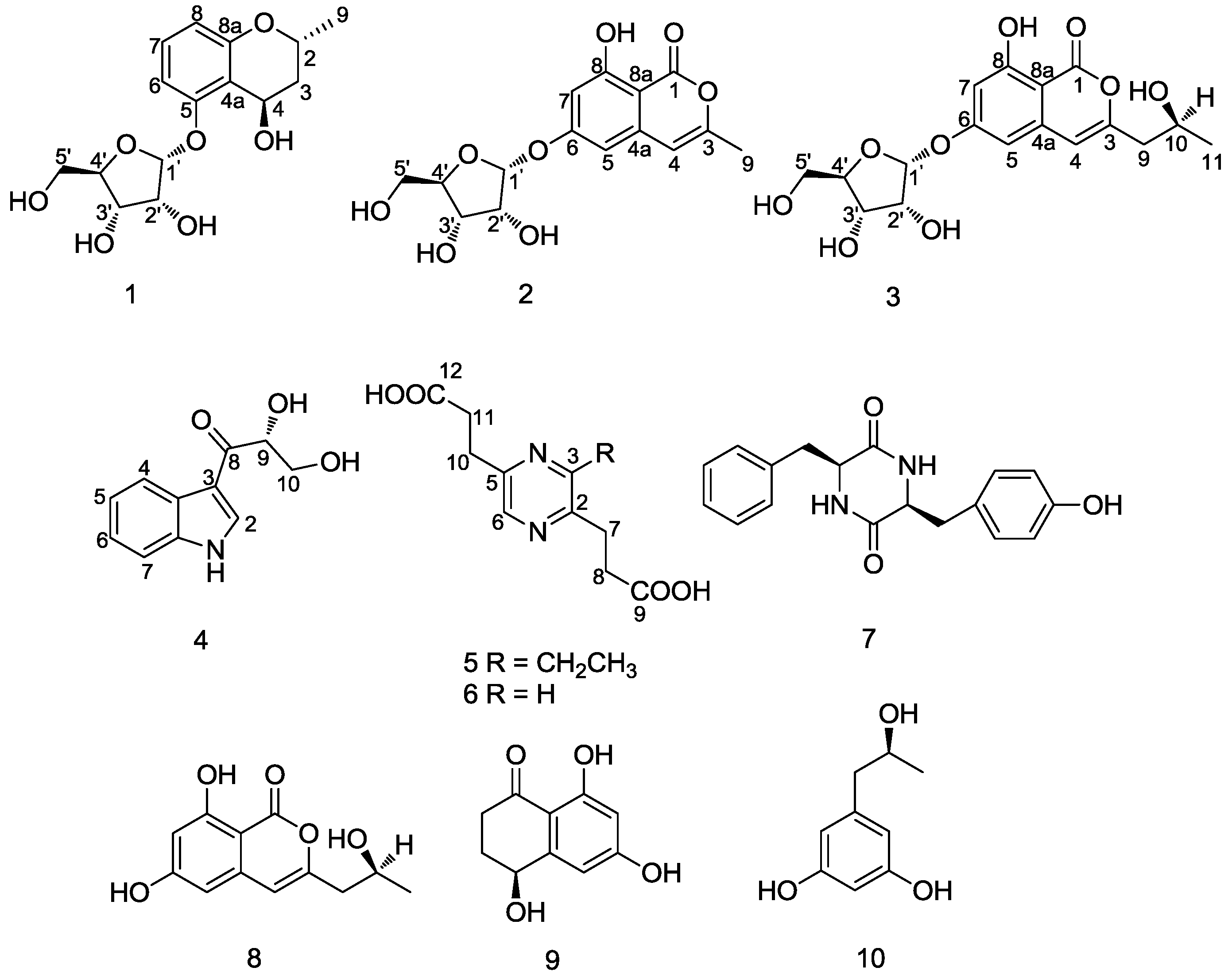
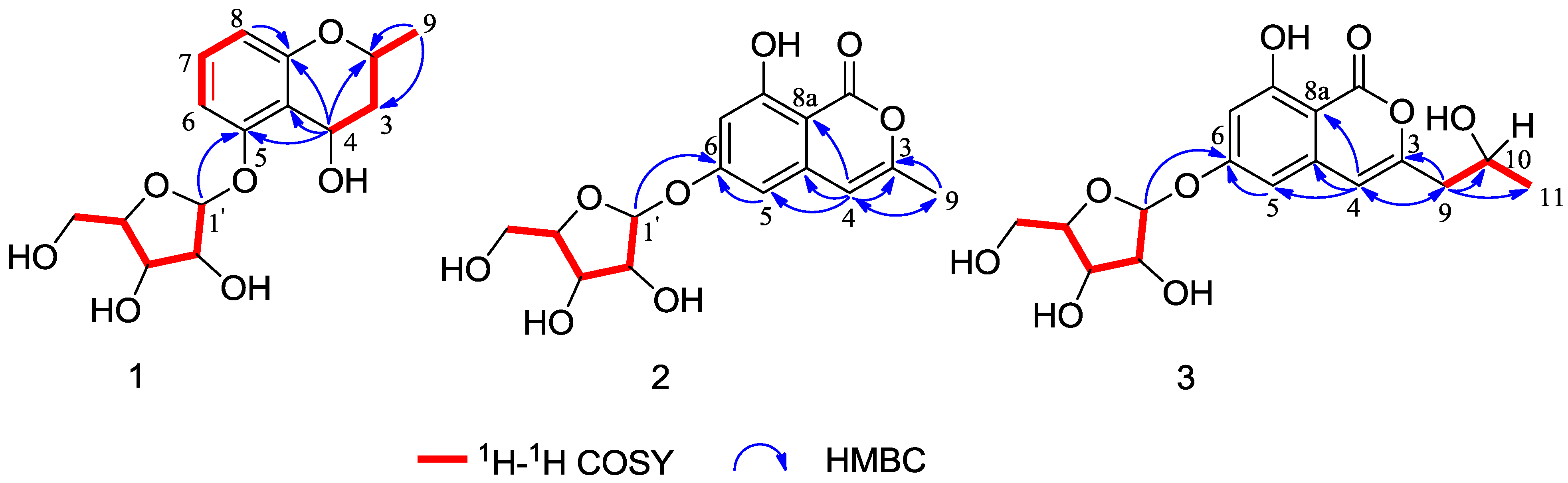
Chemical Structure Elucidation
| NO. | 1 a | 2 a | 3 b | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 167.8 | 167.0 | ||||
| 2 | 4.21, m | 68.8 | ||||
| 3 | 1.68, ddd (4.1,12.0, 14.4) 2.01, dt (1.9, 14.4) | 38.9 | 155.9 | 156.7 | ||
| 4 | 5.04, dd (1.9, 4.1) | 60.1 | 6.31, s | 105.8 | 6.40, s | 106.7 |
| 4a | 116.1 | 141.2 | 140.5 | |||
| 5 | 158.6 | 6.58, d (1.8) | 104.5 | 6.70, d (2.1) | 104.3 | |
| 6 | 6.72, d (8.2) | 107.9 | 166.1 | 165.9 | ||
| 7 | 7.12, t (8.2) | 130.5 | 6.62, d (1.8) | 103.9 | 6.89, d (2.1) | 103.7 |
| 8 | 6.49, d (8.2) | 112.0 | 164.5 | 164.1 | ||
| 8a | 157.4 | 101.4 | 101.5 | |||
| 9 | 1.40, d (6.3) | 21.6 | 2.22, s | 19.4 | 2.67, dd (4.9, 14.3) 2.74, dd (7.8, 14.3) | 44.3 |
| 10 | 4.45, m | 65.4 | ||||
| 11 | 1.40, d (6.2) | 24.4 | ||||
| 1′ | 5.70, d (4.5) | 103.4 | 5.74, d (4.4) | 101.8 | 6.17, d (4.2) | 102.1 |
| 2′ | 4.23, dd (4.5, 6.4) | 73.9 | 4.24, dd (4.4, 6.2) | 73.6 | 4.87, m | 74.1 |
| 3′ | 4.10, dd (2.6, 6.4) | 71.4 | 4.12, dd (3.0, 6.2) | 71.2 | 4.85, m | 71.3 |
| 4′ | 4.20, m | 88.5 | 4.15, m | 88.3 | 4.88, m | 89.0 |
| 5′ | 3.64, dd (3.9, 12.1) 3.69, dd (3.6, 12.1) | 63.5 | 3.65, dd (3.8, 12.1) 3.72, dd (3.3, 12.1) | 63.3 | 4.12, dd (3.8, 12.0) 4.17, dd (3.7, 12.0) | 63.4 |
| NO. | 4 c | 5 d | 6 c | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 12.03, s | |||||
| 2 | 8.41, s | 134.8 | 152.2 | 153.0 | ||
| 3 | 114.1 | 157.2 | 8.44, d (4.1) | 143.3 | ||
| 3a | 125.9 | |||||
| 4 | 8.21, dd (2.0, 6.6) | 121.4 | ||||
| 5 | 7.19, m | 121.9 | 153.9 | 153.0 | ||
| 6 | 7.23, m | 122.9 | 8.25, s | 141.7 | 8.44, d (4.1) | 143.3 |
| 7 | 7.49, dd (1.7, 6.8) | 112.2 | 3.10, t (7.2) | 29.3 | 2.96, t (7.3) | 29.2 |
| 7a | 136.3 | |||||
| 8 | 195.5 | 2.79, t (7.2) | 32.9 | 2.67, t (7.3) | 32.2 | |
| 9 | 4.69, t (4.5) | 75.8 | 177.1 | 173.8 | ||
| 10 | 3.63, dd (5.5, 11.1) 3.71, dd (4.5, 11.1) | 65.3 | 3.04, t (7.2) | 30.7 | 2.96, t (7.3) | 29.2 |
| 11 | 2.76, t (7.2) | 33.9 | 2.67, t (7.3) | 32.2 | ||
| 12 | 176.9 | 173.8 | ||||
| 13 | 2.88, q (7.5) | 28.3 | ||||
| 14 | 1.29, t (7.5) | 13.1 | ||||
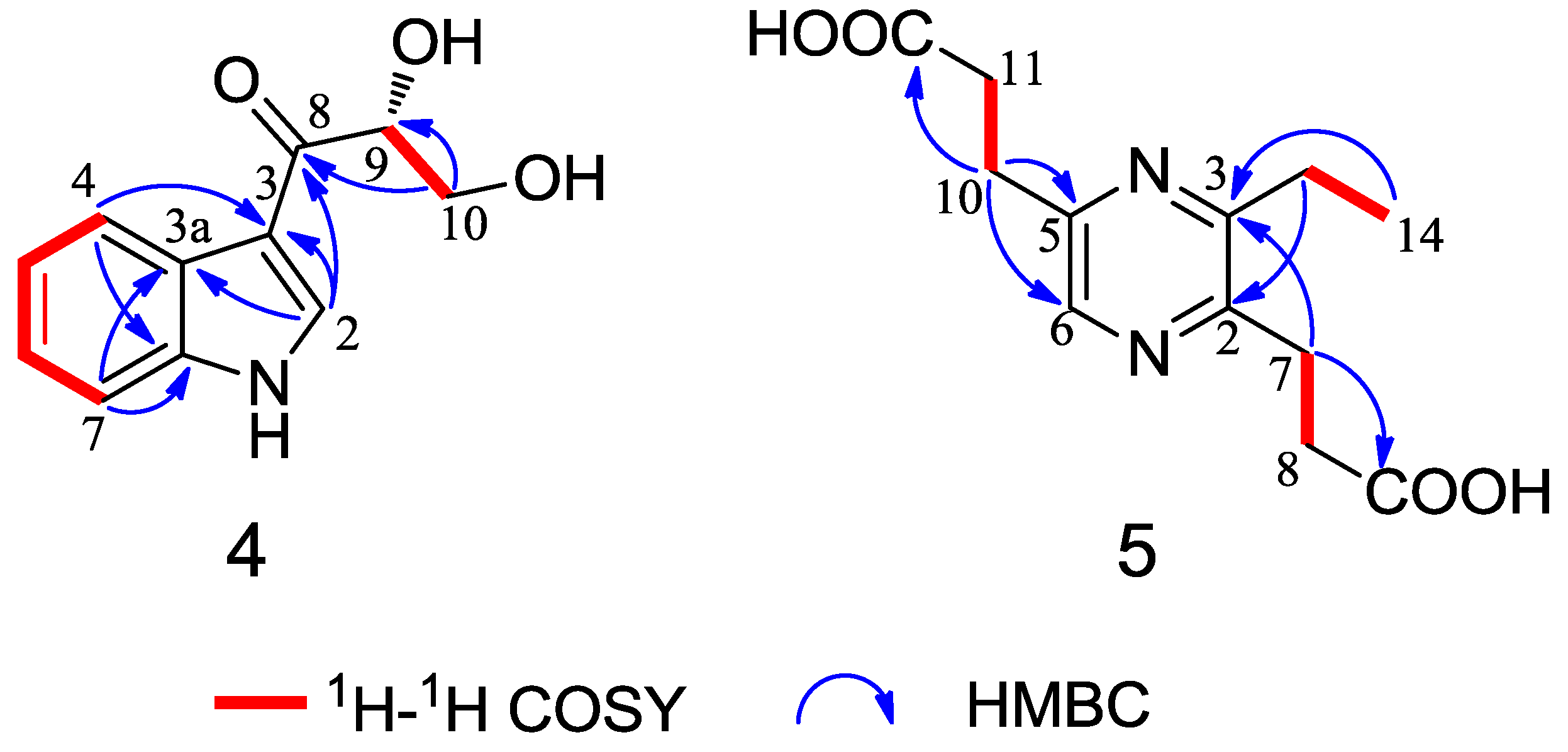

3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation
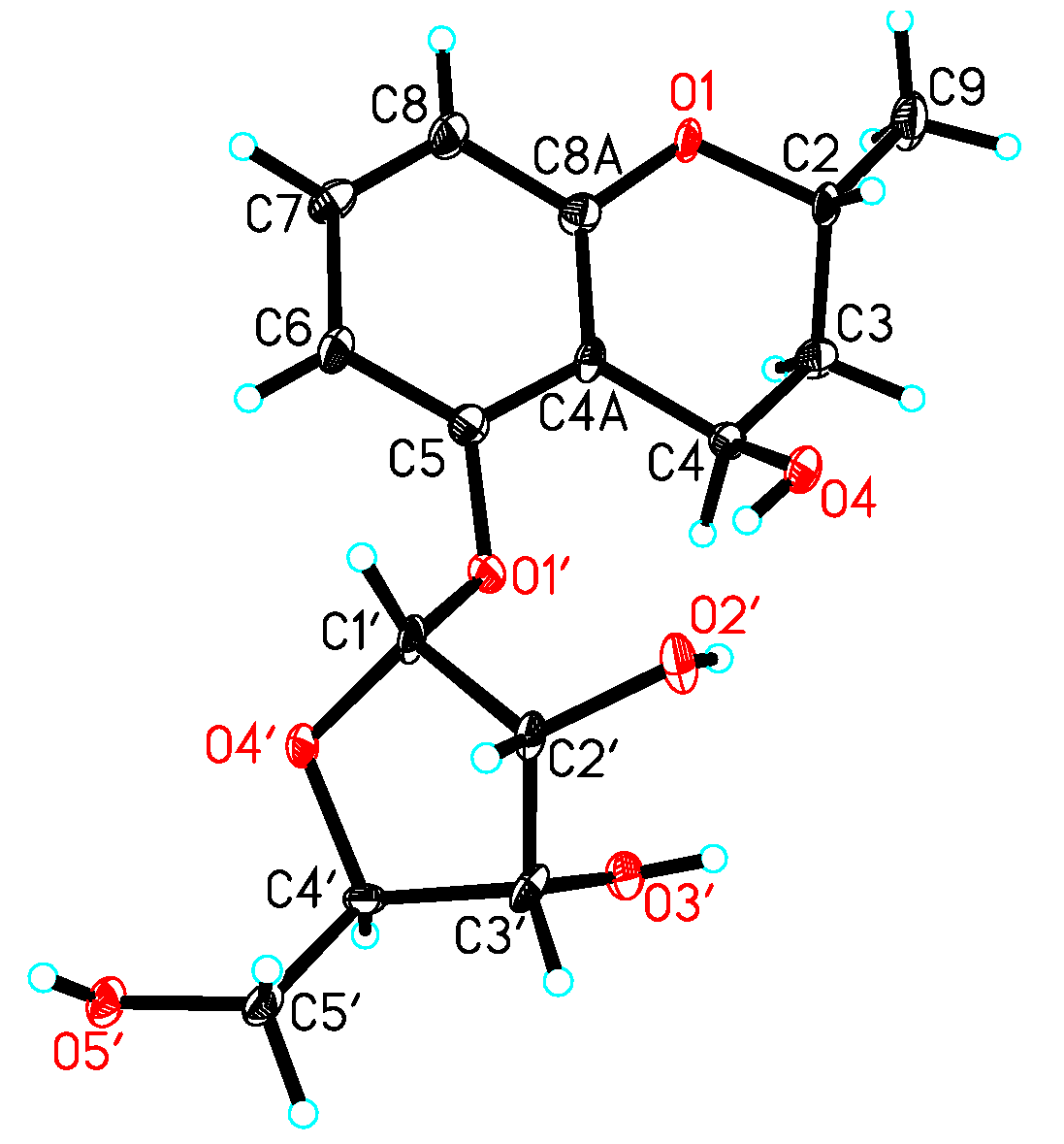
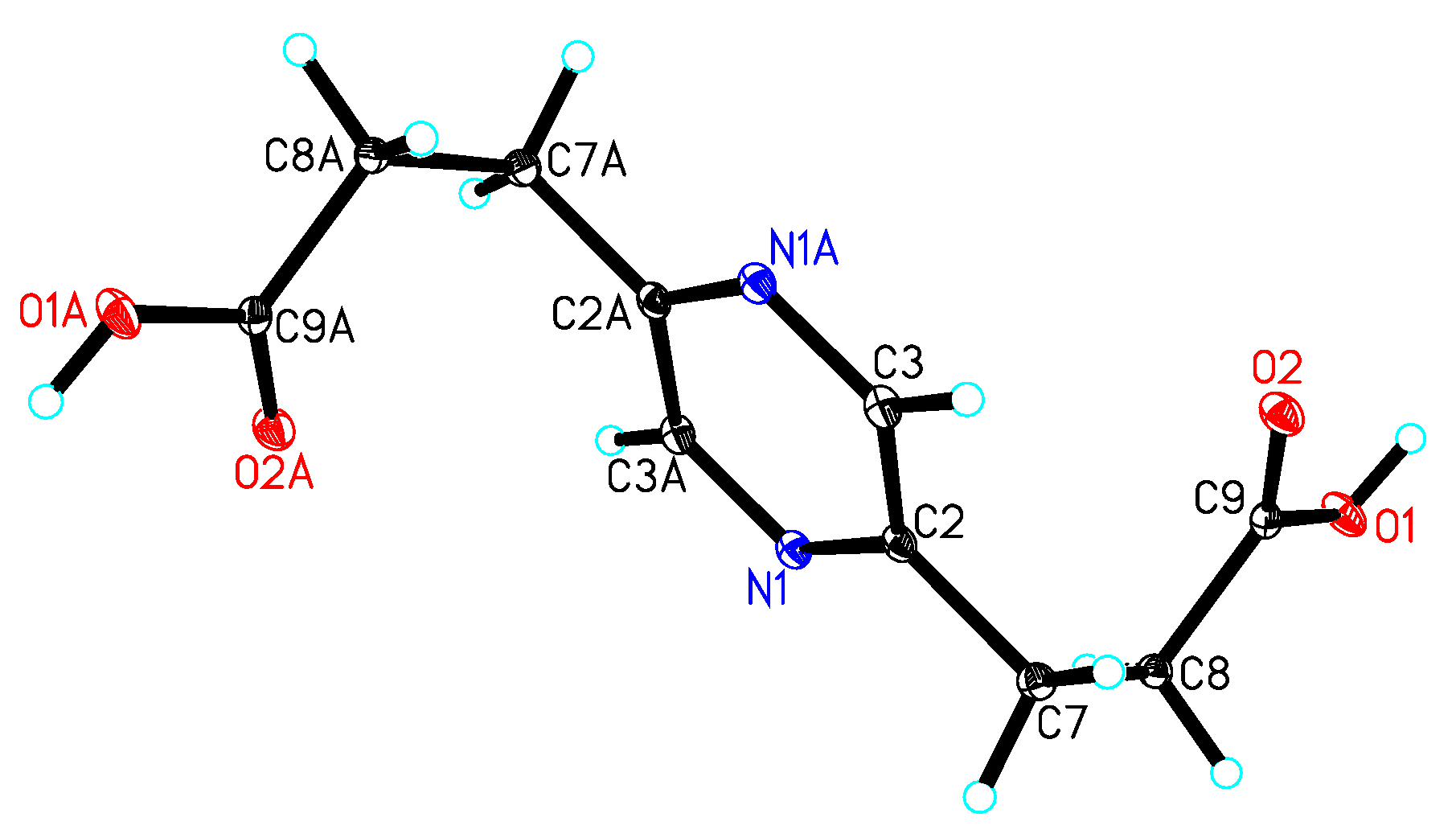
3.4. X-ray Crystallographic Analysis
3.5. Acid hydrolysis and GC Analysis of 1−3 and Determination of the Absolute Configuration of the Sugar Moiety
3.6. Biological Activities
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [PubMed]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [PubMed]
- Zhang, Y.L.; Ge, H.M.; Zhao, W.; Dong, H.; Xu, Q.; Li, S.H.; Li, J.; Zhang, J.; Song, Y.C.; Tan, R.X. Unprecedented immunosuppressive polyketides from Daldinia eschscholzii, a mantis-associated fungus. Angew. Chem. Int. Ed. 2008, 47, 5823–5826. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, J.; Jiang, N.; Lu, Y.H.; Wang, L.; Xu, S.H.; Wang, W.; Zhang, G.F.; Xu, Q.; Ge, H.M.; et al. Immunosuppressive polyketides from mantis-associated Daldinia eschscholzii. J. Am. Chem. Soc. 2011, 133, 5931–5940. [Google Scholar] [CrossRef] [PubMed]
- McCarron, P.A.; Donnelly, R.F.; Woolfson, A.D.; Andrews, G.P. Analysis of pyrazine 2,5-dipropionic acid in 5-aminolevulinic acid-loaded urological and topical delivery vehicles: Methodology and assay validation. J. Pharm. Biomed. Anal. 2005, 36, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Yang, R.Y.; Guo, Z.Y.; She, Z.G.; Lin, Y.C. A new xanthone derivative from mangrove endophytic fungus No. ZSU-H16. Chem. Nat. Compd. 2010, 46, 15–18. [Google Scholar] [CrossRef]
- Hallock, Y.F.; Clardy, J.; Kenfield, D.S.; Strobel, G. De-O-methyldiaporthin, a phytotoxin from Drechslera siccans. Phytochemistry 1988, 27, 3123–3125. [Google Scholar] [CrossRef]
- Dong, J.Y.; Song, H.C.; Li, J.H.; Tang, Y.S.; Sun, R.; Wang, L.; Zhou, Y.P.; Wang, L.M.; Shen, K.Z.; Wang, C.R.; et al. Ymf 1029A-E, preussomerin analogues from the fresh-water-derived fungus YMF 1.01029. J. Nat. Prod. 2008, 71, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, H.; Tenma, M.; Shimazaki, K.; Kobayashi, J. Three new metabolites from the marine yeast Aureobasidium pullulans. J. Nat. Prod. 1998, 61, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, R.U.; Stevens, J.D. The proton magnetic resonance spectra and tautomeric equilibria of aldoses in deuterium oxide. Can. J. Chem. 1966, 44, 249–262. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.M.; Teuscher, F.; Li, D.L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Nhiem, N.X.; Kiem, P.V.; Minh, C.V.; Kim, N.; Park, S.; Lee, H.Y.; Kim, E.S.; Kim, Y.H.; Kim, S.; Koh, Y. Diarylheptanoids and flavonoids from Viscum album inhibit LPS-Stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. J. Nat. Prod. 2013, 76, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- He, W.J.; Chu, H.B.; Zhang, Y.M.; Han, H.J.; Yan, H.; Zeng, G.Z.; Fu, Z.H.; Olubanke, O.; Tan, N.H. Antimicrobial, cytotoxic lignans and terpenoids from the twigs of Pseudolarix Kaempferi. Planta Med. 2011, 77, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Lu, Y.; Liao, Q.; Wu, Y.; Chen, X. Fangchinoline inhibits human immunodeficiency virus type 1 replication by interfering with gp160 proteolytic processing. PLoS One 2012, 7, e39225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Xu, G.L.; Liu, Y.H.; Rao, Y.; Yu, R.Y.; Zhang, Z.W.; Wang, Y.S.; Tao, L. Anti-diabetic activities of Gegen Qinlian Decoction in high-fat diet combined with streptozotocin-induced diabetic rats and in 3T3-L1 adipocytes. Phytomedicine 2013, 20, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.R.; George, S. The nonenzymatic cyclic dimerisation of 5-aminolevulinic acid. Tetrahedron 1992, 48, 7879–7886. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.-X.; Xue, Y.-B.; Bi, X.-B.; Zhang, J.-W.; Luo, Z.-W.; Li, X.-N.; Yao, G.-M.; Wang, J.-P.; Zhang, Y.-H. Five New Secondary Metabolites Produced by a Marine-Associated Fungus, Daldinia eschscholzii. Mar. Drugs 2014, 12, 5563-5575. https://doi.org/10.3390/md12115563
Hu Z-X, Xue Y-B, Bi X-B, Zhang J-W, Luo Z-W, Li X-N, Yao G-M, Wang J-P, Zhang Y-H. Five New Secondary Metabolites Produced by a Marine-Associated Fungus, Daldinia eschscholzii. Marine Drugs. 2014; 12(11):5563-5575. https://doi.org/10.3390/md12115563
Chicago/Turabian StyleHu, Zheng-Xi, Yong-Bo Xue, Xiao-Bin Bi, Jin-Wen Zhang, Zeng-Wei Luo, Xiao-Nian Li, Guang-Min Yao, Jian-Ping Wang, and Yong-Hui Zhang. 2014. "Five New Secondary Metabolites Produced by a Marine-Associated Fungus, Daldinia eschscholzii" Marine Drugs 12, no. 11: 5563-5575. https://doi.org/10.3390/md12115563