Climate Variability and Oceanographic Settings Associated with Interannual Variability in the Initiation of Dinophysis acuminata Blooms
Abstract
:1. Introduction
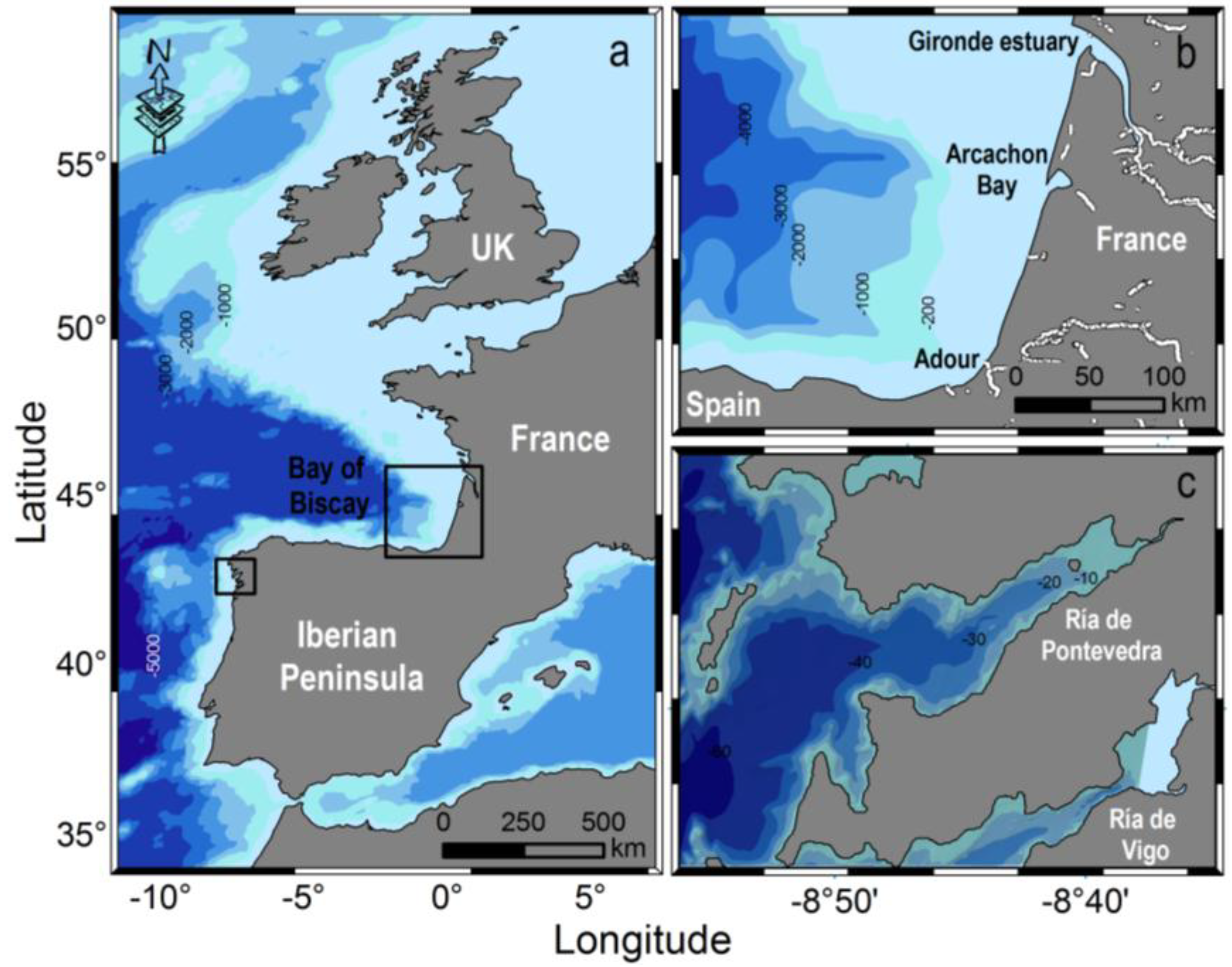
2. Results
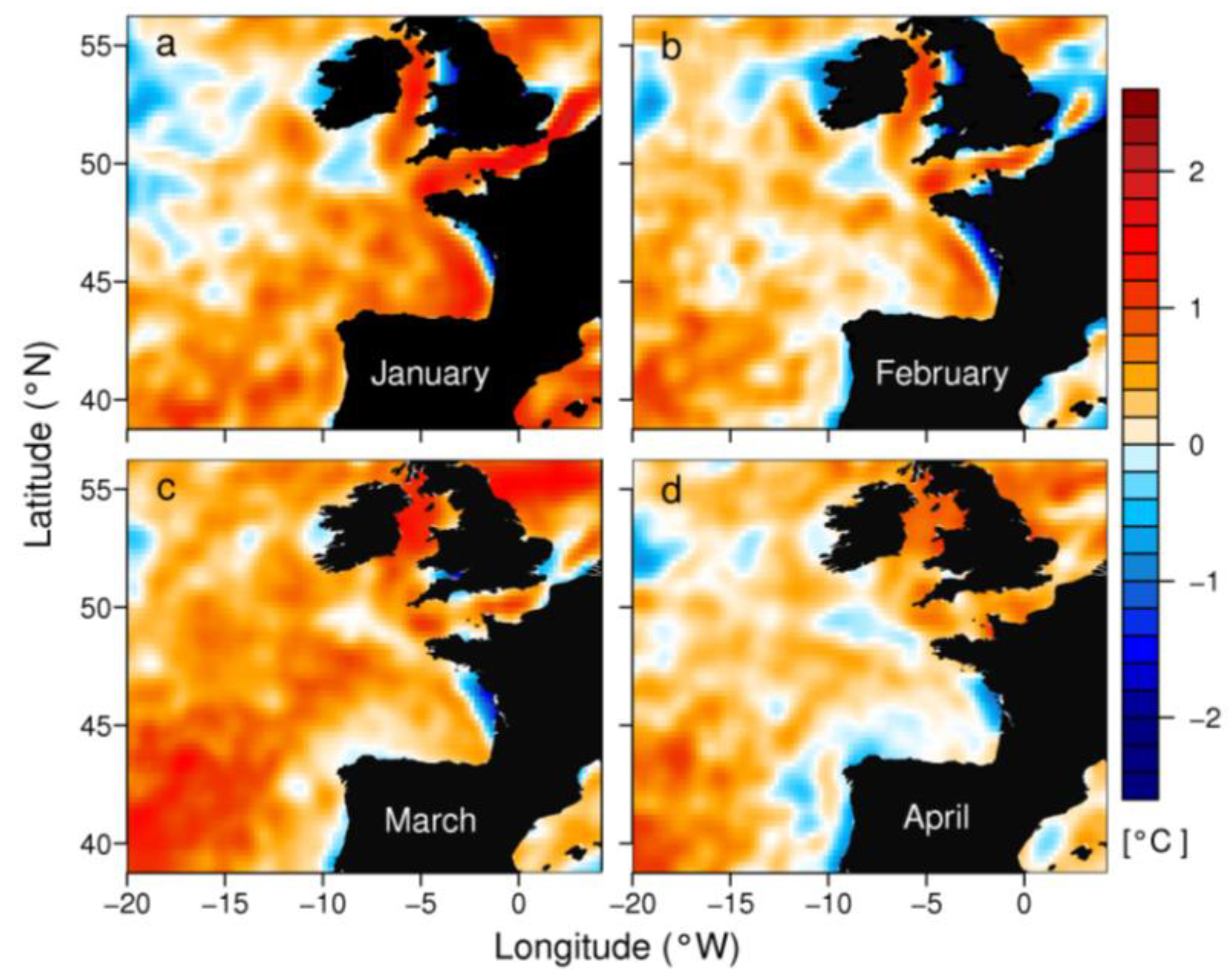
2.1. Meteorological and Hydrodynamic Conditions in the Two Study Areas in 2012
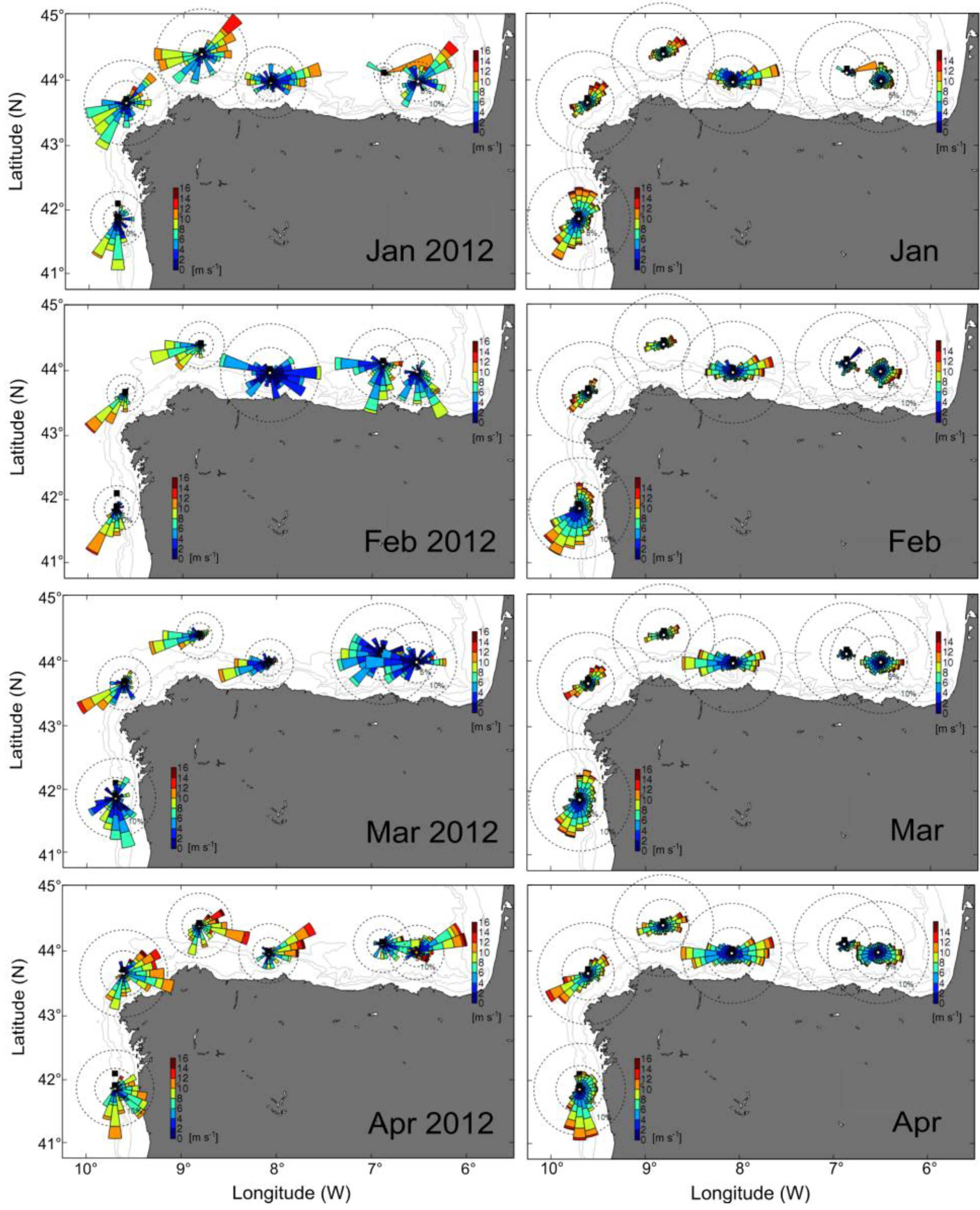


2.2. Distribution of D. acuminata Populations in 2012

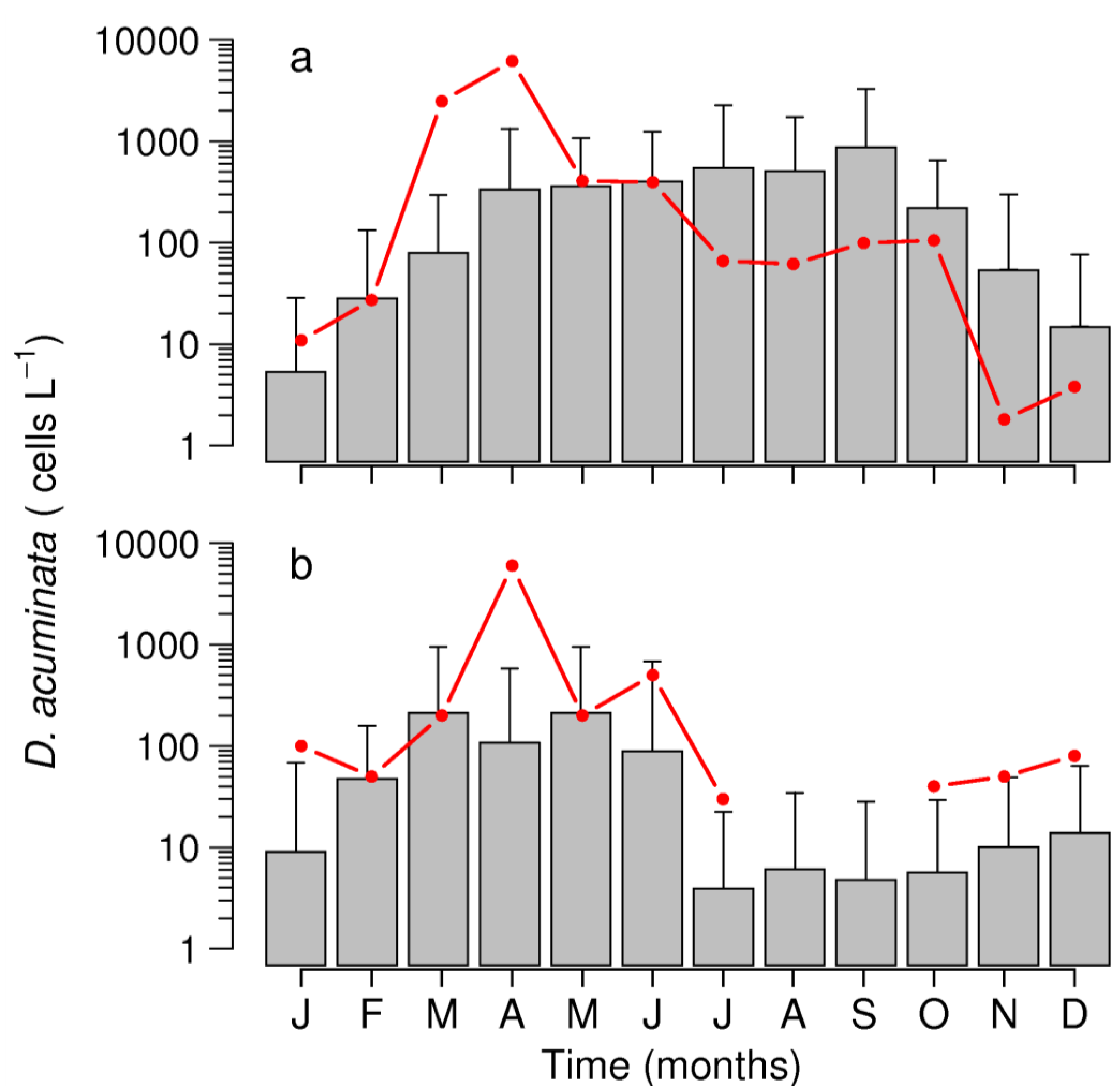
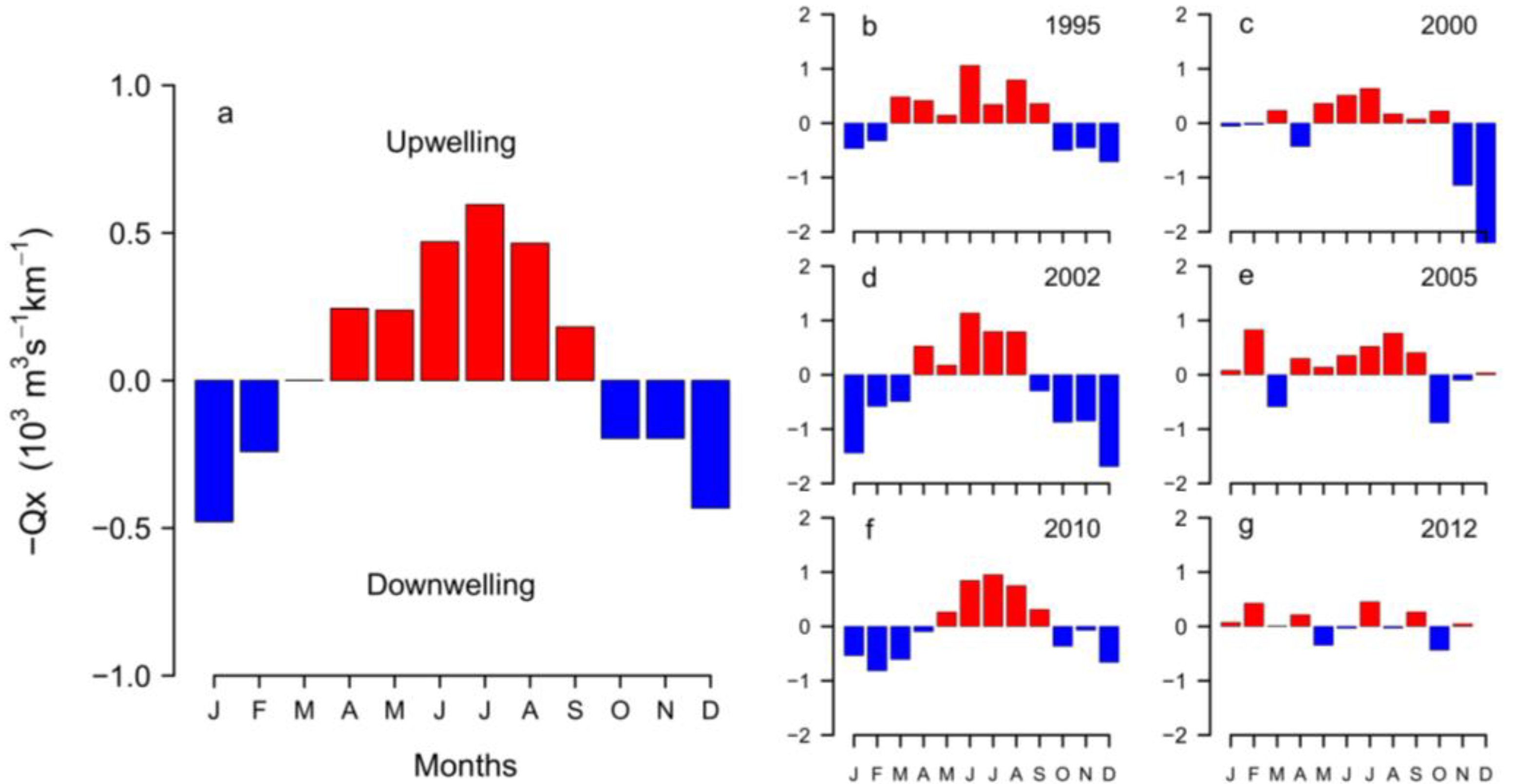
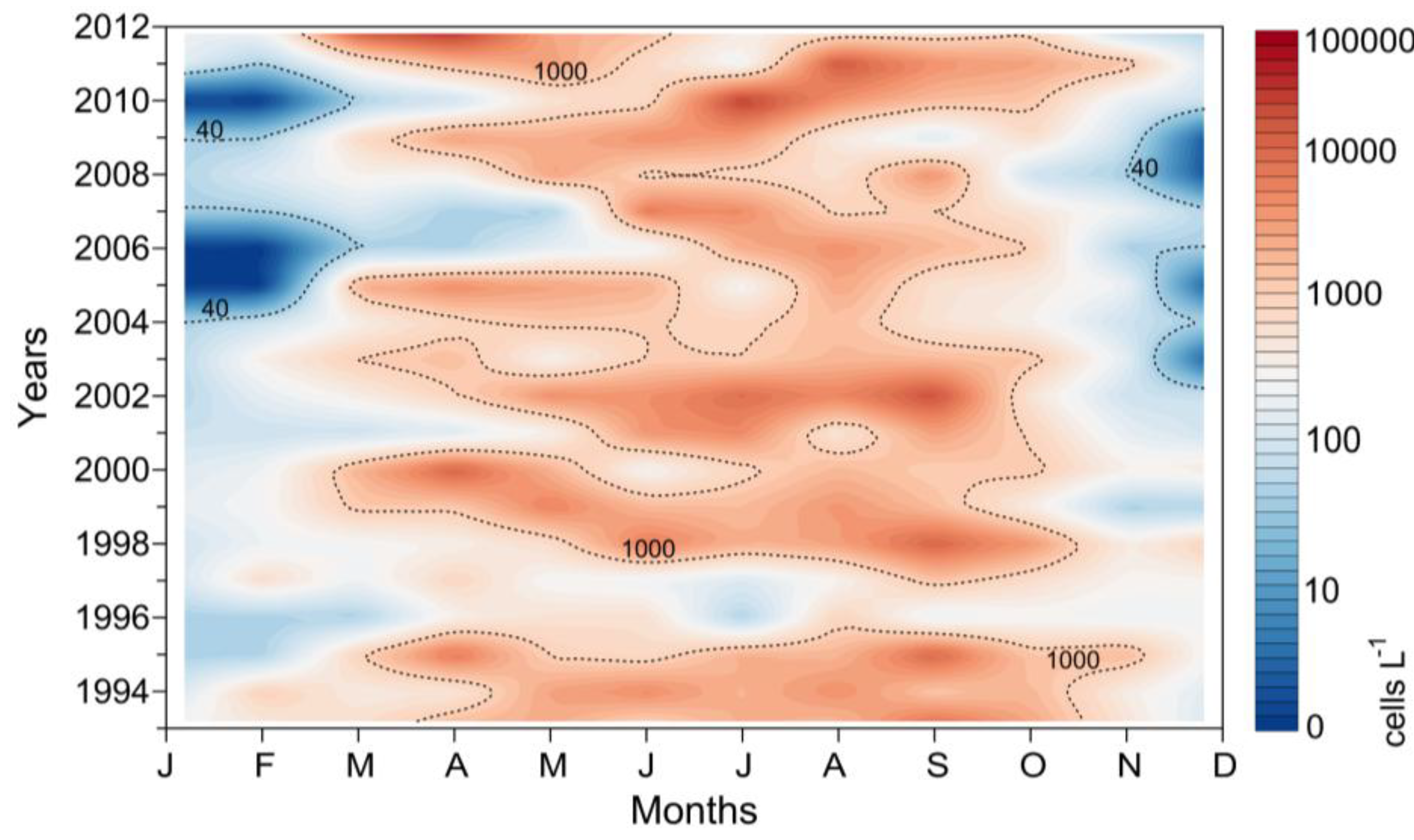
2.3. Interannual Variability of D. acuminata
2.3.1. Ría de Pontevedra (NW Spain)

2.3.2. Arcachon Bay (SW France)

3. Discussion
3.1. Biological Conditions Preceding Dinophysis Blooms
3.2. Anomalous Hydrodynamic Conditions Favouring Initiation/Intensification of D. acuminata Blooms in 2012
3.3. Implications for Predictive Capabilities
4. Experimental Section
4.1. Meteorological Data
4.2. Phytoplankton Data
4.3. Numerical
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M. Harmful Dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- Blanco, J.; Moroño, A.; Fernández, M.L. Toxic episodes in shellfish produced by lipophilic phycotoxins: an overview. Galician J. Mar. Resour. 2005, 1, 70. [Google Scholar]
- Delmas, D.; Herbland, A.; Maestrini, S. Environmental conditions which lead to increase in cell density of the toxic dinoflagellates Dinophysis spp. in nutrient-rich and nutrient-poor waters of the French Atlantic coast. Mar. Ecol. Prog. Ser. 1992, 89, 53–61. [Google Scholar] [CrossRef]
- Lassus, P.; Bardouil, M.; Berthomé, J.P.; Maggi, P.; Truquet, P.; Le Déan, L. Seasonal occurrence of Dinophysis sp. along the French coast between 1983 and 1987. Aquat. Living Resour. 1988, 1, 155–164. [Google Scholar] [CrossRef]
- McMahon, T.; Raine, R.; Silke, J. Oceanographic control of harmful phytoplankton blooms around southwestern Ireland. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and International Oceanographic Commission of UNESCO: Vigo, Spain, 1998; pp. 128–130. [Google Scholar]
- Dahl, E.; Johannessen, T. Relationship between occurrence of Dinophysis species (Dinophyceas) and shellfish toxicity. Phycologia 2001, 40, 223–227. [Google Scholar] [CrossRef]
- Lindahl, O.; Lundve, B.; Johansen, M. Toxicity of Dinophysis spp. in relation to population density and environmental conditions on the Swedish west coast. Harmful Algae 2007, 6, 218–231. [Google Scholar] [CrossRef]
- Robert, R.; Guillocheau, N.; Collos, Y. Hydrobiological parameters during an annual cycle in the Arcachon Basin. Mar. Biol. 1987, 95, 631–640. [Google Scholar] [CrossRef]
- Batifoulier, F.; Lazure, P.; Velo-Suarez, L.; Maurer, D.; Bonneton, P.; Charria, G.; Dupuy, C.; Gentien, P. Distribution of Dinophysis species in the Bay of Biscay and possible transport pathways to Arcachon Bay. J. Mar. Syst. 2012, 109–110, S273–S283. [Google Scholar]
- Glé, C.; Del Amo, Y.; Sautour, B.; Laborde, P.; Chardy, P. Variability of nutrients and phytoplankton primary production in a shallow macrotidal coastal ecosystem (Arcachon Bay, France). Estuar. Coast. Shelf. Sci. 2008, 76, 642–656. [Google Scholar] [CrossRef]
- Labry, C.; Herbland, A.; Delmas, D. The role of phosphorus on planktonic production of the Gironde plume waters in the Bay of Biscay. J. Plankton Res. 2002, 24, 97–117. [Google Scholar] [CrossRef]
- Charria, G.; Lazure, P.; Le Cann, B.; Serpette, A.; Reverdin, G.; Louazel, S.; Batifoulier, F.; Dumas, F.; Pichon, A.; Morel, Y. Surface layer circulation derived from Lagrangian drifters in the Bay of Biscay. J. Mar. Syst. 2013, 109, S60–S76. [Google Scholar]
- Delmas, D.; Herbland, A.; Maestrini, S. Do Dinophysis spp. come from the “open sea” along the French Atlantic coast? In Toxic Phytoplankton Blooms in the Sea; Smayda, T., Shimizu, Y., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1993; pp. 489–494. [Google Scholar]
- Zdanowski, M.K.; Figueiras, F. Relationships between the abundance of bacteria and other biota and the hydrographic variability in the Ría de Vigo, Spain. Mar. Ecol. Prog. Ser. 1997, 147, 257–267. [Google Scholar] [CrossRef]
- Wooster, W.S.; Bakun, A.; McLain, D.R. The seasonal upwelling cycle along the eastern boundary of the North Atlantic. J. Mar. Res. 1976, 34, 131–141. [Google Scholar]
- Tilstone, G.H.; Figueiras, F.G.; Fraga, F. Upwelling-downwelling sequences in the generation of red tides in a coastal upwelling system. Mar. Ecol. Prog. Ser. 1994, 112, 241–253. [Google Scholar] [CrossRef]
- Tilstone, G.H.; Míguez, B.M.; Figueiras, F.G.; Fermín, E.G. Diatom dynamics in a coastal ecosystem affected by upwelling: coupling between species succession, circulation and biogeochemical processes. Mar. Ecol. Prog. Ser. 2000, 205, 23–41. [Google Scholar] [CrossRef]
- Velo-Suárez, L.; González-Gil, S.; Pazos, Y.; Reguera, B. The growth season of Dinophysis acuminata in an upwelling system embayment: A conceptual model based on in situ measurements. Deep Sea Res. II Top. Stud. Oceanogr. 2013. [Google Scholar] [CrossRef]
- Anderson, D.; Stock, C.; Keafer, B.; Nelson, A.; McGillicuddy, D.; Keller, M.; Thompson, B.; Matrai, P.; Martin, J. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep Sea Res. II 2005, 52, 2522–2542. [Google Scholar] [CrossRef]
- Escalera, L.; Reguera, B. Planozygote division and other observations on the sexual cycle of several species of Dinophysis (Dinophyceae, Dinophysiales). J. Phycol. 2008, 44, 1425–1436. [Google Scholar] [CrossRef]
- Smayda, T. Turbulence, watermass stratification and harmful algal blooms: an alternative view and frontal zones as “pelagic seed banks”. Harmful Algae 2002, 1, 95–112. [Google Scholar] [CrossRef]
- Álvarez-Salgado, X.A.; Figueiras, F.G.; Pérez, F.F.; Groom, S.; Nogueira, E.; Borges, A.V.; Chou, L.; Castro, C.G.; Moncoiffé, G.; Ríos, A.F.; Miller, A.E.; Frankignoulle, M.; Savidge, G.; Wollast, R. The Portugal coastal counter current off NW Spain: new insights on its biogeochemical variability. Prog. Oceanogr. 2003, 56, 281–321. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Kim, S.; Kim, H.; Myung, G.; Kang, Y.; Yih, W. First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat. Microb. Ecol. 2006, 45, 101–106. [Google Scholar] [CrossRef]
- Riisgaard, K.; Hansen, J. Role of food uptake for photosynthesis, growth and survival of the mixotrophic dinoflagellate Dinophysis acuminata. Mar. Ecol. Prog. Ser. 2009, 381, 51–62. [Google Scholar] [CrossRef]
- Hansen, P.J.; Nielsen, L.T.; Johnson, M.; Berge, T.; Flynn, K.R. Acquired phototrophy in Mesodinium and Dinophysis—A review of cellular organization, prey selectivity, nutrient uptake and bioenergetics. Harmful Algae 2013. [Google Scholar] [CrossRef]
- Kim, S.; Kang, Y.G.; Kim, H.S.; Yih, W.; Coats, D.W.; Park, M.G. Growth and grazing responses of the mixotrophic dinoflagellate Dinophysis acuminata as functions of light intensity and prey concentration. Aquat. Microb. Ecol. 2008, 51, 301–310. [Google Scholar] [CrossRef]
- González-Gil, S.; Velo-Suárez, L.; Gentien, P.; Ramilo, I.; Reguera, R. Phytoplankton assemblages and characterization of a Dinophysis acuminata population during an upwelling-downwelling cycle. Aquat. Microb. Ecol. 2010, 58, 273–286. [Google Scholar] [CrossRef]
- Villarino, M.L.; Figueiras, F.; Jones, K.; Alvarez-Salgado, X.A.; Richard, J.; Edwards, A. Evidence of in situ diel vertical migration of a red-tide microplancton species in Ría de Vigo (NW Spain). Mar. Biol. 1995, 123, 607–617. [Google Scholar]
- Sjöqvist, C.O.; Lindholm, T.J. Natural co-occurrence of Dinophysis acuminata (Dinoflagellata) and Mesodinium rubrum (Ciliophora) in thin layers in a coastal inlet. J. Eukaryot. Microbiol. 2011, 58, 365–372. [Google Scholar] [CrossRef]
- Gustafson, D.E.; Stoecker, D.K.; Johnson, M.D.; Van Heukelem, W.F.; Sneider, K. Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum. Nature 2000, 405, 1049–1052. [Google Scholar] [CrossRef]
- Koike, K.; Nishiyama, A.; Takishita, K.; Kobiyama, A.; Ogata, T. Appearance of Dinophysis fortii following blooms of certain cryptophyte species. Mar. Ecol. Prog. Ser. 2007, 337, 303–309. [Google Scholar] [CrossRef]
- Reguera, B.; Spanish Institute of Oceanography, Oceanographic Centre of Vigo, Vigo, Spain. unpublished work. 2012.
- Garcia-Soto, C.; Pingree, R.D. Spring and summer blooms of phytoplankton (SeaWiFS/MODIS) along a ferry line in the Bay of Biscay and western English Channel. Cont. Shelf Res. 2009, 29, 1111–1122. [Google Scholar]
- Maurer, D.; Bec, B.; Neaud-Masson, N.; Rumebe, M.; Auby, I.; Grémare, A. Etude des relations entre le phytoplancton et les phénomènes de toxicité d'origine inconnue dans le Bassin d’Arcachon; Ifremer: Arcachon, France, January 2010.
- Puillat, I.; Lazure, P.; Jégou, A.M.; Lampert, L.; Miller, P.I. Hydrographical variability on the French continental shelf in the Bay of Biscay, during the 1990s. Cont. Shelf. Res. 2004, 24, 1143–1163. [Google Scholar] [CrossRef]
- Reverdin, G.; Marié, L.; Lazure, P.; d’Ovidio, F.; Boutin, J.; Testor, P.; Martin, N.; Lourenco, A.; Gaillard, F.; Lavin, A.; et al. Freshwater from the Bay of Biscay shelves in 2009. J. Mar. Syst. 2013, 109–110, S134–S143. [Google Scholar] [CrossRef]
- Masson, D.; Thomas, G.; Genauzeau, S.; Le Moine, O.; IFREMER, La Tremblade, France. Unpublished work. 2012.
- Lasker, R. Field criteria for survival of anchovy larvae: The relation between inshore chlorophyll maximum layers and successful first feeding. Fish. Bull. 1975, 73, 453–462. [Google Scholar]
- Lasker, R. The relation between oceanographic conditions and anchovy food in the California Current: Identification of factors contributing to recruitment failure. Rapp. P.-v. Réun. Cons. int. Explor. Mer. 1978, 173, 212–230. [Google Scholar]
- Alvarez-Fanjul, E.; Alfonso, M.; Ruiz, M.I.; Lopez, J.D.; Rodriguez, I. The deep water network. In Building the European Capacity in Operational Oceanography; Dahlin, H., Ed.; Elsevier: New York, NY, USA, 2003; Volume 445, pp. 398–402. [Google Scholar]
- Lavín, A.; Somavilla, R.; Arteche, J.L.; Rodriguez, C.; Cano, D.; Ruiz-Villarreal, M. The Spanish Institute of Oceanography (IEO) Coastal Observing System at the southern Bay of Biscay, new real-time development: The ocean-meteorological AGL Buoy. In Oceans 2009—Europe; IEEE: New York, NY, USA, 2009; pp. 35–42. [Google Scholar]
- NOAA. Live Access Server (LAS). Available online: http://nomads.ncdc.noaa.gov/las/getUI.do (accessed on 2 July 2013).
- Lindahl, O. A Dividable Hose for Phytoplankton Sampling. Report of the Working Group on Phytoplankton and Management of Their Effects; Technical Report for International Council for the Exploration of the Sea: Hirtshals, Denmark, March 1986.
- Lovegrove, T. An improved form of sedimentation apparatus for use with an inverted microscope. J. Cons. Int. Explor. Mer. 1960, 25, 279–284. [Google Scholar]
- IFREMER. Réseau de Surveillance du Phytoplancton et des Phycotoxines (REPHY). Available online: http://www.ifremer.fr/envlit/surveillance/rephy.htm (accessed on 2 July 2013).
- Uthermöhl, H. Zur Vervollkomnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Limnol. 1958, 9, 1–38. [Google Scholar]
- Lazure, P.; Dumas, F. An external-internal mode coupling for a 3D hydrodynamical model at regional scale (MARS). Adv. Water Resour. 2008, 31, 233–250. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Díaz, P.A.; Reguera, B.; Ruiz-Villarreal, M.; Pazos, Y.; Velo-Suárez, L.; Berger, H.; Sourisseau, M. Climate Variability and Oceanographic Settings Associated with Interannual Variability in the Initiation of Dinophysis acuminata Blooms. Mar. Drugs 2013, 11, 2964-2981. https://doi.org/10.3390/md11082964
Díaz PA, Reguera B, Ruiz-Villarreal M, Pazos Y, Velo-Suárez L, Berger H, Sourisseau M. Climate Variability and Oceanographic Settings Associated with Interannual Variability in the Initiation of Dinophysis acuminata Blooms. Marine Drugs. 2013; 11(8):2964-2981. https://doi.org/10.3390/md11082964
Chicago/Turabian StyleDíaz, Patricio A., Beatriz Reguera, Manuel Ruiz-Villarreal, Yolanda Pazos, Lourdes Velo-Suárez, Henrick Berger, and Marc Sourisseau. 2013. "Climate Variability and Oceanographic Settings Associated with Interannual Variability in the Initiation of Dinophysis acuminata Blooms" Marine Drugs 11, no. 8: 2964-2981. https://doi.org/10.3390/md11082964



