First Identification of 5,11-Dideoxytetrodotoxin in Marine Animals, and Characterization of Major Fragment Ions of Tetrodotoxin and Its Analogs by High Resolution ESI-MS/MS
Abstract
:1. Introduction

2. Results
2.1. LC-MS Chromatographic Properties of Authentic 5,11-DideoxyTTX and 6,11-DideoxyTTX, and Characterization of Their Major Fragment Ions

2.2. Characterization of Major Fragment Ions of TTX and 5,6,11-TrideoxyTTX


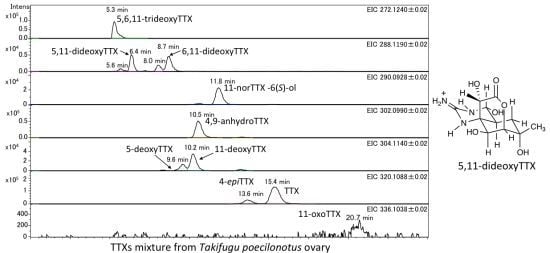
2.3. Identification and Quantitation of 5,11-DideoxyTTX in the Pufferfish, Takifugu poecilonotus, and Flatworm (Planocerid sp. 1)


| Takifugu poecilonotus ovary | Flatworm (planocerid sp. 1) pharynx | |||||
|---|---|---|---|---|---|---|
| Applied amount (Figure 5) (ng) | Contents (µg/g) | Ratio (mol/mol) | Applied amount (Figure 6) (ng) | Contents (µg/g) | Ratio (mol/mol) | |
| TTX | 4.0 | 130 | 100 | 7.0 | 1800 | 100 |
| 4- epiTTX | 0.89 | 30 | 22 | 1.9 | 480 | 27 |
| 4,9-anhydroTTX | 1.1 | 36 | 27 | 1.1 | 280 | 16 |
| 5-deoxyTTX | 0.25 | 8.4 | 6.2 | 0.41 | 100 | 5.9 |
| 11-deoxyTTX | 0.67 | 22 | 17 | 4.0 | 1000 | 57 |
| 6,11-dideoxyTTX | 0.082 | 2.7 | 2.0 | 0.090 | 23 | 1.3 |
| 5,11-dideoxyTTX | 0.065 | 2.2 | 1.6 | 0.66 | 170 | 9.4 |
| 5,6,11-trideoxyTTX | 1.4 | 46 | 34 | 3.1 | 770 | 44 |
| 11-norTTX-6(S)-ol | 0.49 | 17 | 12 | 8.4 | 2100 | 120 |
| 11-oxoTTX | 0.0072 | 0.24 | 0.18 | 0.026 | 6.6 | 0.37 |
2.4. Quantitation of 5,11-DideoxyTTX in the Ovary and Liver of Three Female Specimens of the Pufferfish, Takifugu pardalis
| Contents (µg/g) | ||
|---|---|---|
| ovary ( n = 3) | liver ( n = 3) | |
| TTX | 5.5–27 | 12–35 |
| 4- epiTTX | 0.34–3.4 | 1.8–3.0 |
| 4,9-anhydroTTX | 3.1–12 | 9.7–16 |
| 5-deoxyTTX | 0.66–1.0 | <0.056–1.4 |
| 11-deoxyTTX | 0.32–3.4 | 0.15–0.84 |
| 6,11-dideoxyTTX | 0.95–6.8 | 1.0–11 |
| 5,11-dideoxyTTX | 0.25–0.64 | 0.26–0.35 |
| 5,6,11-trideoxyTTX | 6.6–35 | 0.59–0.87 |
| 11-norTTX-6( S)-ol | <0.074–5.2 | <0.056–0.30 |
| 11-oxoTTX | <0.074 | <0.056 |
2.5. Toxicity of 5,11-DideoxyTTX to Mice
3. Discussion

4. Experimental Section
4.1. Authentic (−)-5,11-DideoxyTTX, TTX, 6,11-DideoxyTTX and 5,6,11-TrideoxyTTX
4.2. Preparation of Sample Solutions of Pufferfish and Flatworm for LC-MS Analysis
4.3. High Resolution ESI-LC-MS and LC-MS/MS
5. Conclusions
Acknowledgment
Conflict of Interest
References
- Tsuda, K.; Ikuma, S.; Kawamura, M.; Tachikawa, R.; Sakai, K.; Tamura, C.; Amakasu, O. Tetrodotoxin. VII. On the structure of tetrodotoxin and its derivatives. Chem. Pharm. Bull. 1964, 12, 1357–1374. [Google Scholar] [CrossRef]
- Woodward, R.B. The structure of tetrodotoxin. Pure Appl. Chem. 1964, 9, 49–74. [Google Scholar] [CrossRef]
- Goto, T.; Kishi, Y.; Takahashi, S.; Hirata, Y. Tetrodotoxin. Tetrahedron 1965, 21, 2059–2088. [Google Scholar] [CrossRef]
- Narahashi, T.; Deguchi, T.; Urakawa, N.; Ohkubo, Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. Am. J. Physiol. 1960, 198, 934–938. [Google Scholar]
- Kao, C.Y. Tetrodotoxin, saxitoxin, and the molecular biology of the sodium channel. Ann. N. Y. Acad. Sci. 1986, 479, 1–14. [Google Scholar] [CrossRef]
- Yokoo, A. Chemical studies on tetrodotoxin Rept. III. Isolation of spheroidine. J. Chem. Soc. Japan 1950, 71, 591–592. [Google Scholar]
- Noguchi, T.; Uzu, A.; Koyama, K.; Hashimoto, K. Occurrence of tetrodotoxin as the major toxin in xanthid crab Atergatis floridus. Bull. Jpn. Soc. Sci. Fish. 1983, 49, 1887–1892. [Google Scholar] [CrossRef]
- Yasumura, D.; Oshima, Y.; Yasumoto, T.; Alcala, A.C.; Alcala, L.C. Tetrodotoxin and paralytic shellfish toxins in Philippine crabs. Agric. Biol. Chem. 1986, 50, 593–598. [Google Scholar] [CrossRef]
- Miyazawa, K.; Jeon, J.K.; Maruyama, J.; Noguchi, T.; Ito, K.; Hashimoto, K. Occurrence of tetrodotoxin in the flatworm Planocera multitentaculata. Toxicon 1986, 24, 645–650. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Yotsu-Yamashita, M.; Paul, V. Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc. Natl. Acad. Sci. USA 2006, 103, 3176–3179. [Google Scholar]
- Noguchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Harada, T. Occurrence of tetrodotoxin in the Japanese ivory shell Babylonia japonica. Bull. Japan. Soc. Sci. Fish. 1981, 47, 901–913. [Google Scholar] [CrossRef]
- Maruyama, J.; Noguchi, T.; Narita, H.; Jeon, J.K.; Otsuka, M.; Hashimoto, K. Occurrence of tetrodotoxin in a starfish, Astropecten scoparius. Agric. Biol. Chem. 1985, 49, 3069–3070. [Google Scholar] [CrossRef]
- Sheumack, D.D.; Howden, M.E.H.; Spence, I.; Quinn, R.J. Maculotoxin: A Neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin. Science 1978, 199, 188–189. [Google Scholar]
- McNabb, P.; Selwood, A.I.; Munday, R.; Wood, S.A.; Taylor, D.I.; MacKenzie, A.L.; van Ginkel, R.; Rhodes, L.L.; Cornelisen, C.; Heasman, K.; Patrick, T.; Holland, C.K. Detection of tetrodotoxin from the grey sided-gilled sea slug-Pleurobranchaea maculata, and associated dog neurotoxicosis on beaches adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon 2010, 56, 466–473. [Google Scholar] [CrossRef]
- Fuhrman, F.A.; Fuhrman, G.J.; Mosher, H.S. Toxin from skin of frogs of the genus Atelopus: differentiation from Dendrobatid toxins. Science 1969, 165, 1376–1379. [Google Scholar]
- Buchwald, H.D.; Durham, L.; Fischer, H.G.; Harada, R.; Mosher, H.S.; Kao, C.Y.; Fuhrman, F.A. Identity of tarichatoxin and tetrodotoxin. Science 1964, 143, 474–475. [Google Scholar]
- Yasumoto, T.; Yasumura, D.; Yotsu, M.; Michishita, T.; Endo, A.; Kotaki, Y. Bacterial production of tetrodotoxin and anhydrotetrodotoxin. Agric. Biol. Chem. 1986, 50, 793–795. [Google Scholar] [CrossRef]
- Noguchi, T.; Jeo, J.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of a xanthid crab, Atergatis floridus. J. Biochem. 1986, 99, 311–314. [Google Scholar]
- Kono, M.; Matsui, T.; Furukaw, K.; Yotsu-Yamashita, M.; Yamamori, K. Accumulation of tetrodotoxin and 4,9-anhydrotetrodotoxin in cultured juvenile kusafugu Fugu niphobles by dietary administration of natural toxic komonfugu Fugu poecilonotus liver. Toxicon 2008, 51, 1269–1273. [Google Scholar] [CrossRef]
- Tatsuno, R.; Shikina, M.; Shirai, Y.; Wang, J.; Soyano, K.; Nishihara, J.N.; Takatani, T.; Arakawa, O. Change in the transfer profile of orally administered tetrodotoxin to non-toxic cultured pufferfish Takifugu rubripes depending of its development stage. Toxicon 2013, 65, 76–80. [Google Scholar] [CrossRef]
- Kono, M.; Matsui, T.; Furukawa, K.; Takase, T.; Yamamori, K.; Kaneda, H.; Aoki, D.; Jang, J.H.; Yotsu-Yamashita, M. Examination of transformation among tetrodotoxin and its analogs in the living cultured juvenile puffer fish, kusafugu, Fugu niphobles by intramuscular administration. Toxicon 2008, 52, 714–720. [Google Scholar] [CrossRef]
- Ikeda, K.; Murakami, Y.; Emoto, Y.; Ngy, L.; Taniyama, S.; Yagi, M.; Takatani, T.; Arakawa, O. Transfer profile of intramuscularly administered tetrodotoxin to non-toxic cultured specimens of the pufferfish Takifugu rubripes. Toxicon 2009, 53, 99–103. [Google Scholar] [CrossRef]
- Wang, J.; Araki, T.; Tatsuno, R.; Nina, S.; Ikeda, K.; Hamasaki, M.; Sakakura, Y.; Takatani, T.; Arakawa, O. Transfer profile of intramuscularly administered tetrodotoxin to artificial hybrid specimens of pufferfish, Takifugu rubripes and Takifugu niphobles. Toxicon 2011, 58, 565–569. [Google Scholar] [CrossRef]
- Hanifin, C.T. The chemical and evolutionary ecology of tetrodotoxin (TTX) toxicity in terrestrial vertebrates. Mar. Drugs 2010, 8, 577–593. [Google Scholar] [CrossRef]
- Yasumoto, T.; Yotsu-Yamashita, M. Chemical and etiological studies on tetrodotoxin and its analogs. J. Toxicol. Toxin Rev. 1996, 15, 81–90. [Google Scholar]
- Yotsu-Yamashita, M. Chemistry of puffer fish toxin. J. Toxicol. Toxin Rev. 2001, 20, 51–66. [Google Scholar] [CrossRef]
- Nakamura, M.; Yasumoto, T. Tetrodotoxin derivatives in puffer fish. Toxicon 1985, 23, 271–276. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Schimmele, B.; Yasumoto, T. Isolation and structural assignment of 5-deoxytetrodotoxin from the puffer fish Fugu poecilonotus. Biosci. Biotechnol. Biochem. 1999, 63, 961–963. [Google Scholar] [CrossRef]
- Jang, J.; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987. [Google Scholar] [CrossRef]
- Yasumoto, T.; Yotsu, M.; Murata, M.; Naoki, H. New tetrodotoxin analogue from the newt Cynops ensicauda. J. Am. Chem. Soc. 1988, 110, 2344–2345. [Google Scholar] [CrossRef]
- Jang, J.H.; Yotsu-Yamashita, M. 6,11-DideoxyTTX from the puffer fish, Fugu pardalis. Toxicon 2007, 50, 947–951. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Yamagishi, Y.; Yasumoto, T. 5,6,11-Trideoxytetrodotoxin from the puffer fish, Fugu poecilonotus. Tetrahedron Lett. 1995, 36, 9329–9332. [Google Scholar] [CrossRef]
- Khora, S.S.; Yasumoto, T. Isolation of 11-oxotetrodotoxin from the puffer Arothron nigropunctatus. Tetrahedron Lett. 1989, 30, 4393–4394. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Hayashi, Y.; Khora, S.S.; Sato, S.; Yasumoto, T. Isolation and structural assignment of 11-nortetrodotoxin-6(S)-ol from the puffer Arothron nigropunctatus. Biosci. Biotechnol. Biochem. 1992, 56, 370–371. [Google Scholar] [CrossRef]
- Endo, A.; Khora, S.S.; Murata, M.; Yasumoto, T. Isolation of 11-nortetrodotoxin-6(R)-ol and other tetrodotoxin derivatives from the puffer Fugu niphobles. Tetrahedron Lett. 1988, 29, 4127–4128. [Google Scholar] [CrossRef]
- Yotsu, M.; Yasumoto, T.; Kim, Y.H.; Kao, C.Y. The structure of chiriquitoxin from the Costa Rican frog Atelopus chiriquiensis. Tetrahedron Lett. 1990, 31, 3187–3190. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Tateki, E. First report on toxins in the Panamanian toads Atelopus limosus, A. glyphus and A. certus. Toxicon 2010, 55, 153–156. [Google Scholar] [CrossRef]
- Kotaki, Y.; Shimizu, Y. 1-Hydroxy-5,11-dideoxytetrodotoxin, the first N-hydroxy and ringdeoxy derivative of tetrodotoxin found in the newt Taricha granulosa. J. Am. Chem. Soc. 1993, 115, 827–830. [Google Scholar] [CrossRef]
- Kudo, Y.; Yasumoto, T.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Isolation and structural determination of the first 8-epi-type tetrodotoxin analogs from the newt, Cynops ensicauda popei, and comparison of tetrodotoxin analogs profiles of this newt and the puffer fish, Fugu poecilonotus. Mar. Drugs 2012, 10, 655–667. [Google Scholar] [CrossRef]
- Nzoughet, J.K.; Campbell, K.; Barnes, P.; Cooper, K.M.; Chevallier, O.P.; Elliott, C.T. Comparison of sample preparation methods, validation of an UPLC-MS/MS procedure for the quantification of tetrodotoxin present in marine gastropods and analysis of pufferfish. Food Chem. 2013, 136, 1584–1589. [Google Scholar] [CrossRef]
- Nakagawa, T.; Jang, J.; Yotsu-Yamashita, M. Hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006, 352, 142–144. [Google Scholar] [CrossRef]
- Shoji, Y.; Yotsu-Yamashita, M.; Miyazawa, T.; Yasumoto, T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001, 290, 10–17. [Google Scholar] [CrossRef]
- Jang, J.; Lee, J.S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Jang, J.H.; Cho, Y.; Konoki, K. Optimization of simultaneous analysis of tetrodotoxin, 4-epitetrodotoxin, 4,9-anhydrotetrodotoxin, and 5,6,11-trideoxytetrodotoxin by hydrophilic interaction liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2011, 29, 61–64. [Google Scholar] [CrossRef]
- Nishikawa, T.; Asai, M.; Ohyabu, N.; Yamamoto, N.; Isobe, M. Stereocontrolled Synthesis of (−)-5,11-Dideoxytetrodotoxin. Angew. Chem. Int. Ed. 1999, 38, 3081–3083. [Google Scholar] [CrossRef]
- Asai, M.; Nishikawa, T.; Ohyabu, N.; Yamamoto, N.; Isobe, M. Stereocontrolled Synthesis of (−)-5,11-Dideoxytetrodotoxin. Tetrahedron 2001, 57, 4543–4558. [Google Scholar] [CrossRef]
- Nishikawa, T.; Isobe, M. Synthesis of tetrodotoxin, a classic but still fascinating natural product. Chem. Rec. 2013, 13, 286–302. [Google Scholar] [CrossRef]
- Isobe, M.; Suwan, S.; Franz, T. Recent progress in ultramicroanalysis by LC-Q-TOF. JASCO Report 2000, 42, 1–18. [Google Scholar]
- Adachi, M.; Imazu, T.; Isobe, M.; Nishikawa, T. An improved synthesis of (−)-5,11-dideoxytetrodotoxin. J. Org. Chem. 2013, 78, 1699–1705. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Sugimoto, A.; Takai, A.; Yasumoto, T. Effects of specific modifications of several hydroxyls of tetrodotoxin on its affinity to rat brain membrane. J. Pharmacol. Exp. Ther. 1999, 289, 1688–1696. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yotsu-Yamashita, M.; Abe, Y.; Kudo, Y.; Ritson-Williams, R.; Paul, V.J.; Konoki, K.; Cho, Y.; Adachi, M.; Imazu, T.; Nishikawa, T.; et al. First Identification of 5,11-Dideoxytetrodotoxin in Marine Animals, and Characterization of Major Fragment Ions of Tetrodotoxin and Its Analogs by High Resolution ESI-MS/MS. Mar. Drugs 2013, 11, 2799-2813. https://doi.org/10.3390/md11082799
Yotsu-Yamashita M, Abe Y, Kudo Y, Ritson-Williams R, Paul VJ, Konoki K, Cho Y, Adachi M, Imazu T, Nishikawa T, et al. First Identification of 5,11-Dideoxytetrodotoxin in Marine Animals, and Characterization of Major Fragment Ions of Tetrodotoxin and Its Analogs by High Resolution ESI-MS/MS. Marine Drugs. 2013; 11(8):2799-2813. https://doi.org/10.3390/md11082799
Chicago/Turabian StyleYotsu-Yamashita, Mari, Yuka Abe, Yuta Kudo, Raphael Ritson-Williams, Valerie J. Paul, Keiichi Konoki, Yuko Cho, Masaatsu Adachi, Takuya Imazu, Toshio Nishikawa, and et al. 2013. "First Identification of 5,11-Dideoxytetrodotoxin in Marine Animals, and Characterization of Major Fragment Ions of Tetrodotoxin and Its Analogs by High Resolution ESI-MS/MS" Marine Drugs 11, no. 8: 2799-2813. https://doi.org/10.3390/md11082799
APA StyleYotsu-Yamashita, M., Abe, Y., Kudo, Y., Ritson-Williams, R., Paul, V. J., Konoki, K., Cho, Y., Adachi, M., Imazu, T., Nishikawa, T., & Isobe, M. (2013). First Identification of 5,11-Dideoxytetrodotoxin in Marine Animals, and Characterization of Major Fragment Ions of Tetrodotoxin and Its Analogs by High Resolution ESI-MS/MS. Marine Drugs, 11(8), 2799-2813. https://doi.org/10.3390/md11082799