Total Synthesis of Fellutamide B and Deoxy-Fellutamides B, C, and D
Abstract
:1. Introduction

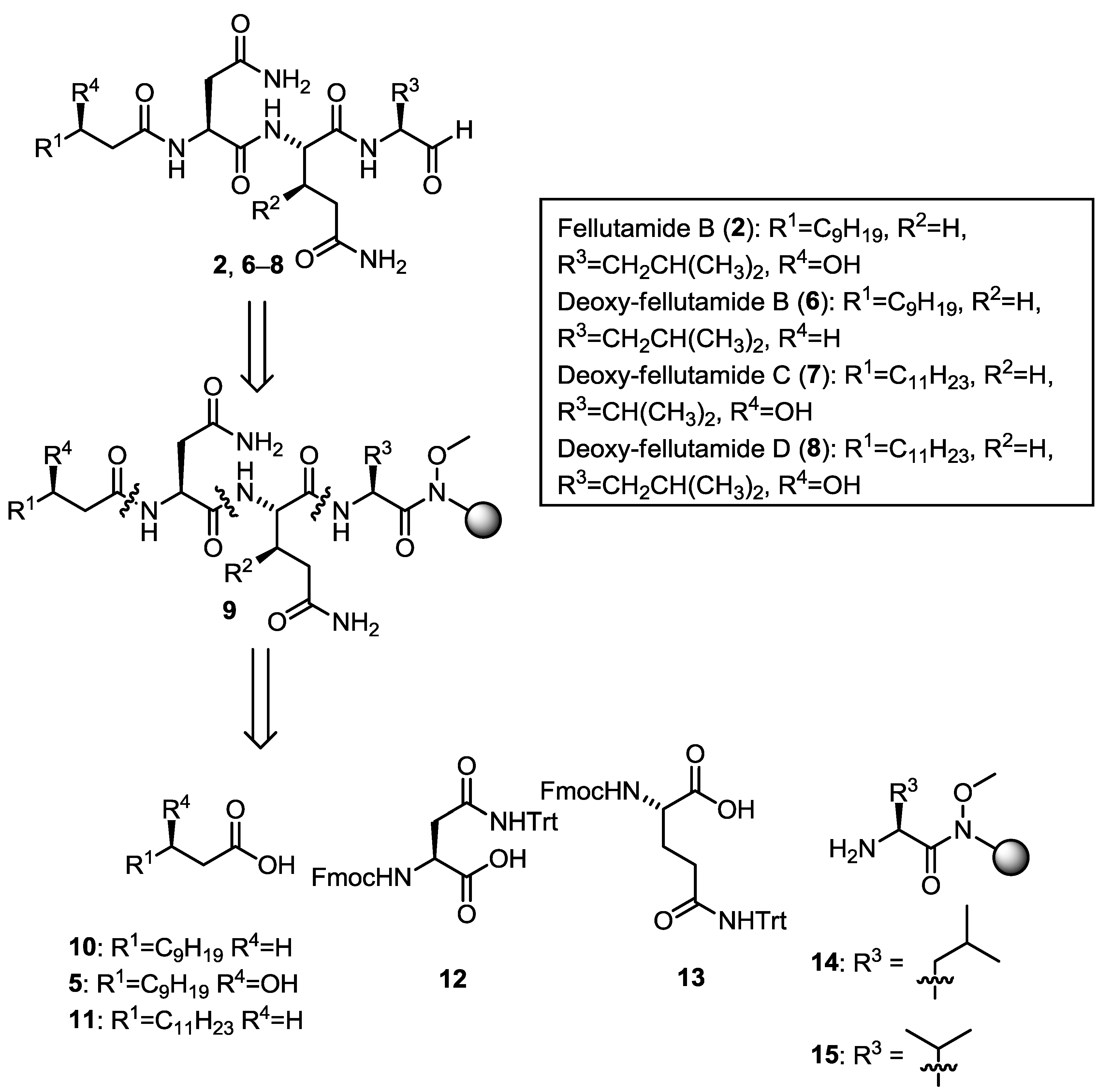
2. Results and Discussion
2.1. Synthetic Plan
Synthesis of (3R)-Hydroxy Lauric Acid (5)


2.2. Total Synthesis of Fellutamide B (2) and Deoxy-Fellutamides B (6), C (7), and D (8)
| Compound | R1 | R3 | R4 | Yield (%) |
|---|---|---|---|---|
| Fellutamide B (2) | C9H19 | CH2CH(CH3)2 | OH | 55 |
| Deoxyfellutamide B (6) | C9H19 | CH2CH(CH3)2 | H | 44 (24% after purification) |
| Deoxyfellutamide C (7) | C11H23 | CH(CH3)2 | OH | 53 |
| Deoxyfellutamide D (8) | C11H23 | CH2CH(CH3)2 | OH | 60 |
2.3. Testing of Fellutamide B (2) and Deoxy-Fellutamides B (6), C (7), and D (8) against Mycobacterium tuberculosis
3. Experimental Section
3.1. General Experimental Methods
3.2. (3R)-Hydroxy Lauric Acid (5)
3.2.1. (4R)-Tridec-1-en-4-ol (16)
3.2.2. (3R)-3-Hydroxydodecanoic Acid (5)
3.3. Mosher’s Ester Determination of Enantiomeric Excess
3.3.1. Methyl (3R)-3-Hydroxydodecanoate (Methyl Ester-5)
3.3.2. Tridec-1-en-4-ol (rac-16)
3.3.3. 3-Hydroxydodecanoic Acid (rac-5)
3.3.4. Methyl 3-Hydroxydodecanoate (rac-Methyl Ester 5)
3.3.5. General Procedure for Mosher’s Esterification
3.4. Solid Phase Peptide Synthesis (0.1 mmol)
3.5. Resin Cleavage Conditions (0.1 mmol)
3.6. Synthesis of Peptides 2 and 6–8
3.6.1. Fellutamide B (2)
3.6.2. Deoxy-fellutamide B (6)
3.6.3. Deoxy-fellutamide C (7)
3.6.4. Deoxy-fellutamide D (8)
3.7. Mycobacterium tuberculosis Inhibition Assays
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Shigemori, H.; Wakuri, S.; Yazawa, K.; Nakamura, T.; Sasaki, T.; Kobayashi, J. Fellutamide-A and fellutamide-B, cytotoxic peptides from a marine fish-possessing fungus Penicillium fellutanum. Tetrahedron 1991, 47, 8529–8534. [Google Scholar] [CrossRef]
- Schneekloth, J.S., Jr.; Sanders, J.L.; Hines, J.; Crews, C.M. Neurotrophic peptide aldehydes: Solid phase synthesis of fellutamide B and a simplified analog. Bioorg. Med. Chem. Lett. 2006, 16, 3855–3858. [Google Scholar] [CrossRef]
- Xu, D.; Ondeyka, J.; Harris, G.H.; Zink, D.; Kahn, J.N.; Wang, H.; Bills, G.; Platas, G.; Wang, W.; Szewczak, A.A.; et al. Isolation, structure, and biological activities of fellutamides C and D from an undescribed Metulocladosporiella (chaetothyriales) using the genome-wide Candida albicans fitness test. J. Nat. Prod. 2011, 74, 1721–1730. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tsuji, T.; Wakuri, S.; Yazawa, K.; Kondo, K.; Shigemori, H.; Kobayashi, J. Stimulation of nerve growth-factor synthesis and secretion by fellutamide-A in vitro. Biosci. Biotechnol. Biochem. 1993, 57, 195–199. [Google Scholar] [CrossRef]
- Lin, G.; Li, D.; Chidawanyika, T.; Nathan, C.; Li, H. Fellutamide B is a potent inhibitor of the Mycobacterium tuberculosis proteasome. Arch. Biochem. Biophys. 2010, 501, 214–220. [Google Scholar] [CrossRef]
- Lin, G.; Li, D.Y.; de Carvalho, L.P.S.; Deng, H.T.; Tao, H.; Vogt, G.; Wu, K.Y.; Schneider, J.; Chidawanyika, T.; Warren, J.D.; et al. Inhibitors selective for mycobacterial versus human proteasomes. Nature 2009, 461, 621–626. [Google Scholar] [CrossRef]
- Nahm, S.; Weinreb, S.M. N-Methoxy-N-methylamides as effective acylating agents. Tetrahedron Lett. 1981, 22, 3815–3818. [Google Scholar] [CrossRef]
- Fehrentz, J.A.; Paris, M.; Heitz, A.; Velek, J.; Liu, C.F.; Winternitz, F.; Martinez, J. Improved solid-phase synthesis of C-terminal peptide aldehydes. Tetrahedron Lett. 1995, 36, 7871–7874. [Google Scholar] [CrossRef]
- Dinh, T.Q.; Armstrong, R.W. Synthesis of ketones and aldehydes via reactions of Weinreb-type amides on solid support. Tetrahedron Lett. 1996, 37, 1161–1164. [Google Scholar] [CrossRef]
- Bauer, J.; Brandenburg, K.; Zahringer, U.; Rademann, J. Chemical synthesis of a glycolipid library by a solid-phase strategy allows elucidation of the structural specificity of immunostimulation by rhamnolipids. Chem. Eur. J. 2006, 12, 7116–7124. [Google Scholar] [CrossRef]
- Guaragna, A.; Nisco, M.D.; Pedatella, S.; Palumbo, G. Studies towards lipid A: A synthetic strategy for the enantioselective preparation of 3-hydroxy fatty acids. Tetrahedron Asymmetry 2006, 17, 2839–2841. [Google Scholar] [CrossRef]
- Brown, H.C.; Jadhav, P.K. Asymmetric carbon-carbon bond formation via β-allyldiisopinocampheylborane. Simple synthesis of secondary homoallylic alcohols with excellent enantiomeric purities. J. Am. Chem. Soc. 1983, 105, 2092–2093. [Google Scholar] [CrossRef]
- Zhu, S.F.; Qiao, X.C.; Zhang, Y.Z.; Wang, L.X.; Zhou, Q.L. Highly enantioselective palladium-catalyzed umpolung allylation of aldehydes. Chem. Sci. 2011, 2, 2428–2428. [Google Scholar] [CrossRef]
- Dale, J.A.; Dull, D.L.; Mosher, H.S. α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines. J. Org. Chem. 1969, 34, 2543–2549. [Google Scholar] [CrossRef]
- Nakahata, M.; Imaida, M.; Ozaki, H.; Harada, T.; Tai, A. The preparation of optically pure 3-hydroxyalkanoic acid—The enantioface-differentiating hydrogenation of the C=O double-bond with modified raney-nickel. Bull. Chem. Soc. Jpn. 1982, 55, 2186–2189. [Google Scholar] [CrossRef]
- Darwin, K.H.; Ehrt, S.; Gutierrez-Ramos, J.C.; Weich, N.; Nathan, C.F. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 2003, 302, 1963–1966. [Google Scholar] [CrossRef]
- Bryk, R.; Gold, B.; Venugopal, A.; Singh, J.; Samy, R.; Pupek, K.; Cao, H.; Popescu, C.; Gurney, M.; Hotha, S.; et al. Selective killing of nonreplicating mycobacteria. Cell Host Microbe 2008, 3, 137–145. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 5th ed; Elsevier: Waltham, MA, USA, 2003. [Google Scholar]
- Hasdemir, B.; Onar, H.C.; Yusufoglu, A. Asymmetric synthesis of long chain β-hydroxy fatty acid methyl esters as new elastase inhibitors. Tetrahedron Asymmetry 2012, 23, 1100–1105. [Google Scholar] [CrossRef]
- Dommisse, A.; Wirtz, J.; Koch, K.; Barthlott, W.; Kolter, T. Synthesis of (S)-nonacosan-10-ol, the major component of tubular plant wax crystals. Eur. J. Org. Chem. 2007, 21, 3508–3511. [Google Scholar]
- Skogh, M. The higher normal chain dl-β-hydroxy acids—Synthesis and investigation of the crystal behaviour of 17 homologous acids with 8 to 24 carbon atoms. Acta Chem. Scand. 1952, 6, 809–817. [Google Scholar] [CrossRef]
- Taneja, N.K.; Tyagi, J.S. Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J. Antimicrob. Chemother. 2007, 60, 288–293. [Google Scholar] [CrossRef]
- Manos-Turvey, A.; Cergol, K.M.; Salam, N.K.; Bulloch, E.M.M.; Chi, G.; Pang, A.; Britton, W.J.; West, N.P.; Baker, E.N.; Lott, J.S.; et al. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org. Biomol. Chem. 2012, 10, 9223–9236. [Google Scholar] [CrossRef]
- West, N.P.; Cergol, K.M.; Xue, M.; Randall, E.J.; Britton, W.J.; Payne, R.J. Inhibitors of an essential mycobacterial cell wall lipase (Rv3802c) as tuberculosis drug leads. Chem. Commun. 2011, 47, 5166–5168. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Giltrap, A.M.; Cergol, K.M.; Pang, A.; Britton, W.J.; Payne, R.J. Total Synthesis of Fellutamide B and Deoxy-Fellutamides B, C, and D. Mar. Drugs 2013, 11, 2382-2397. https://doi.org/10.3390/md11072382
Giltrap AM, Cergol KM, Pang A, Britton WJ, Payne RJ. Total Synthesis of Fellutamide B and Deoxy-Fellutamides B, C, and D. Marine Drugs. 2013; 11(7):2382-2397. https://doi.org/10.3390/md11072382
Chicago/Turabian StyleGiltrap, Andrew M., Katie M. Cergol, Angel Pang, Warwick J. Britton, and Richard J. Payne. 2013. "Total Synthesis of Fellutamide B and Deoxy-Fellutamides B, C, and D" Marine Drugs 11, no. 7: 2382-2397. https://doi.org/10.3390/md11072382




