Cloning, Characterization and Heterologous Expression of the Indolocarbazole Biosynthetic Gene Cluster from Marine-Derived Streptomyces sanyensis FMA
Abstract
:1. Introduction

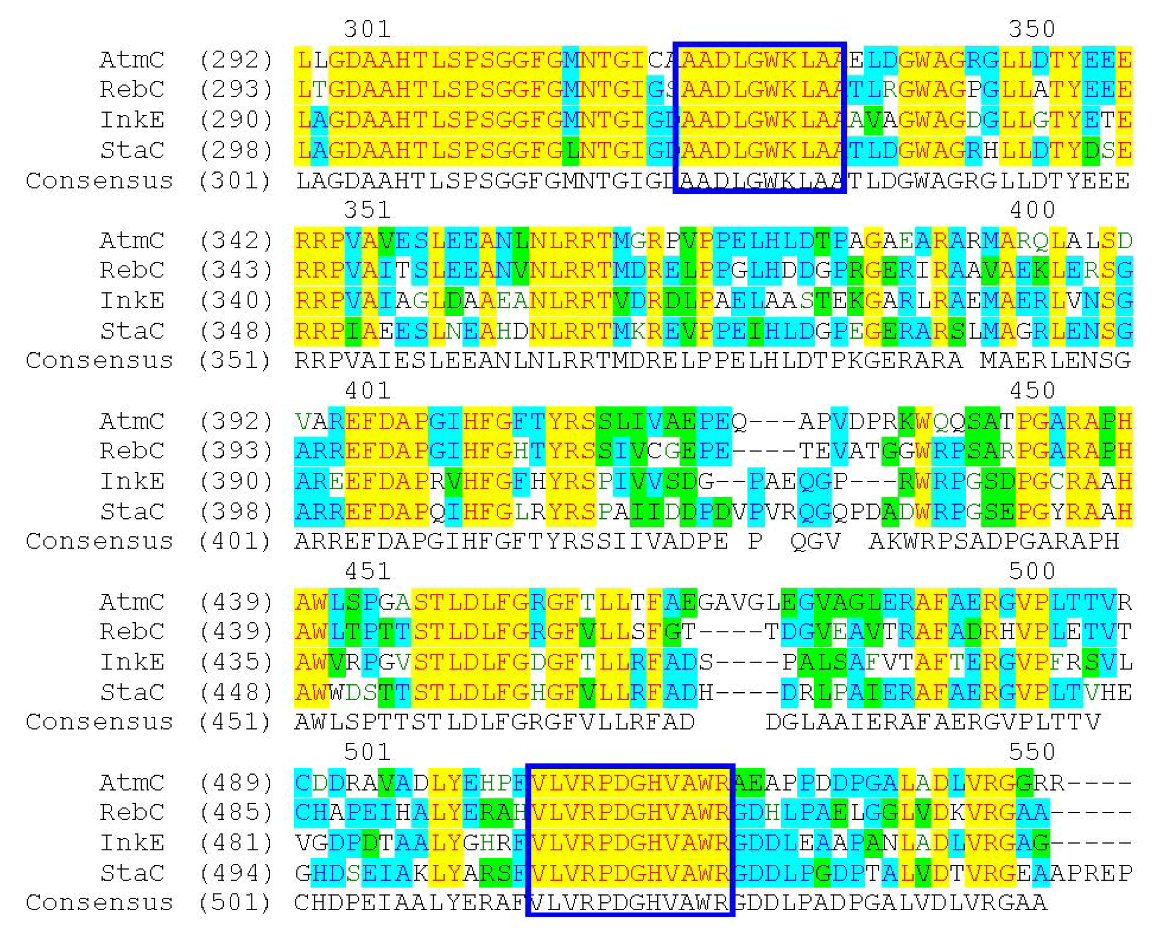
2. Results and Discussion
2.1. Cloning and Sequencing of the spc Gene Cluster from S. sanyensis FMA
2.2. Organization and Characterization of the spc Gene Cluster
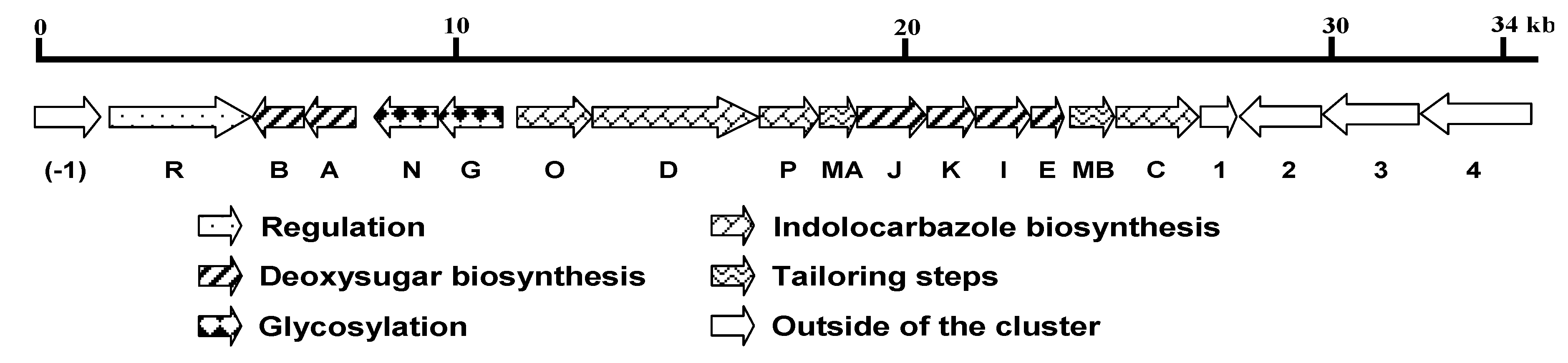
| Protein | Size (aa) | Proposed function | Homolog in strain TP-A0274 (Accession No.) | % Identity/Similarity |
|---|---|---|---|---|
| Orf(-1) | 341 | hypothetical protein | - | - |
| SpcR | 986 | transcriptional regulator | StaR (BAC55205.1) | 62/70% |
| SpcB | 357 | dTDP-glucose 4,6-dehydratase | StaB (BAC55206.1) | 82/87% |
| SpcA | 354 | glucose-1-phosphate thymidyltransferase | StaA (BAC55207.1) | 78/87% |
| SpcN | 390 | cytochrome P450 | StaN (BAC55208.1) | 76/81% |
| SpcG | 433 | N-glycosyltransferase | StaG (BAC55209.1) | 76/84% |
| SpcO | 503 | L-amino acid oxidase | StaO (BAC55210.1) | 77/85% |
| SpcD | 1123 | chromopyrrolic acid synthase | StaD (BAC55211.1) | 65/71% |
| SpcP | 427 | cytochrome P450 | StaP (BAC55212.1) | 71/79% |
| SpcMA | 277 | methyltransferase | StaMA (BAC55213.1) | 63/72% |
| SpcJ | 477 | 2,3-dehydratase | StaJ (BAC55214.1) | 64/70% |
| SpcK | 331 | 4-ketoreductase | StaK (BAC55215.1) | 75/83% |
| SpcI | 369 | aminotransferase | StaI (BAC55216.1) | 86/92% |
| SpcE | 208 | 3,5-epimerase | StaE (BAC55217.1) | 83/89% |
| SpcMB | 281 | methyltransferase | StaMB (BAC55218.1) | 75/83% |
| SpcC | 542 | monooxygenase | StaC (BAF47693.1) | 78/86% |
| Orf1 | 238 | hypothetical protein | - | - |
| Orf2 | 540 | integral membrane protein | - | - |
| Orf3 | 292 | outer membrane adhesion like protein | - | - |
| Orf4 | 660 | integral membrane protein | - | - |
| Orf5 | 884 | D-alanyl-D-alanine carboxypeptidase | - | - |

2.3. Involvement of the spc Gene Cluster in ICZs Biosynthesis in S. sanyensis FMA
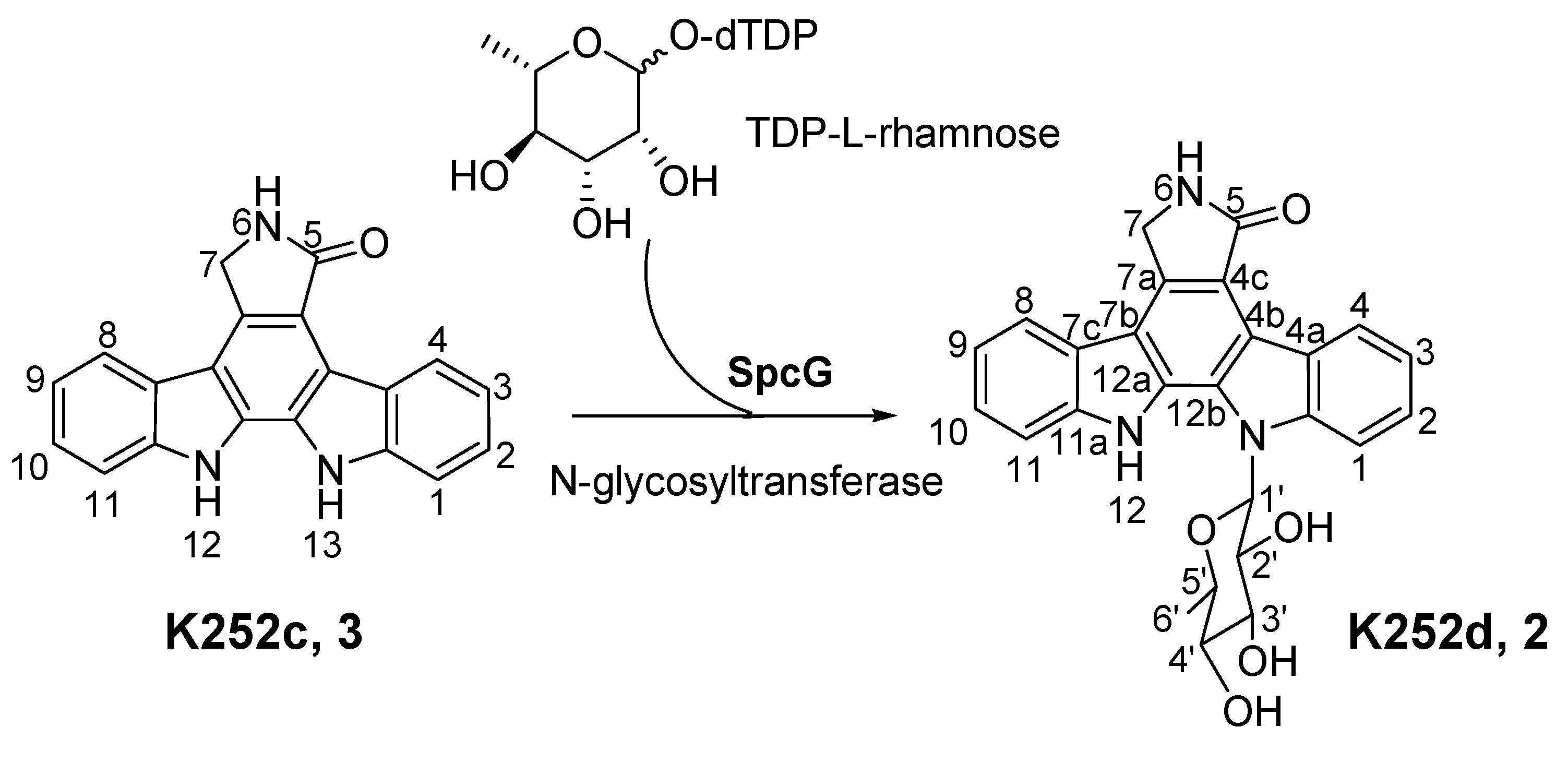
2.4. Heterologous Expression of the spc Gene Cluster in Streptomyces coelicolor M1152
3. Experimental Section
3.1. Bacterial Strains, Plasmids and Reagents
3.2. DNA Manipulation, Sequencing and Bioinformatic Analysis
3.3. Genomic Library Construction
3.4. Library Screening
3.5. Gene Inactivation
3.6. Heterologous Expression of the spc Gene Cluster in S. coelicolor M1152
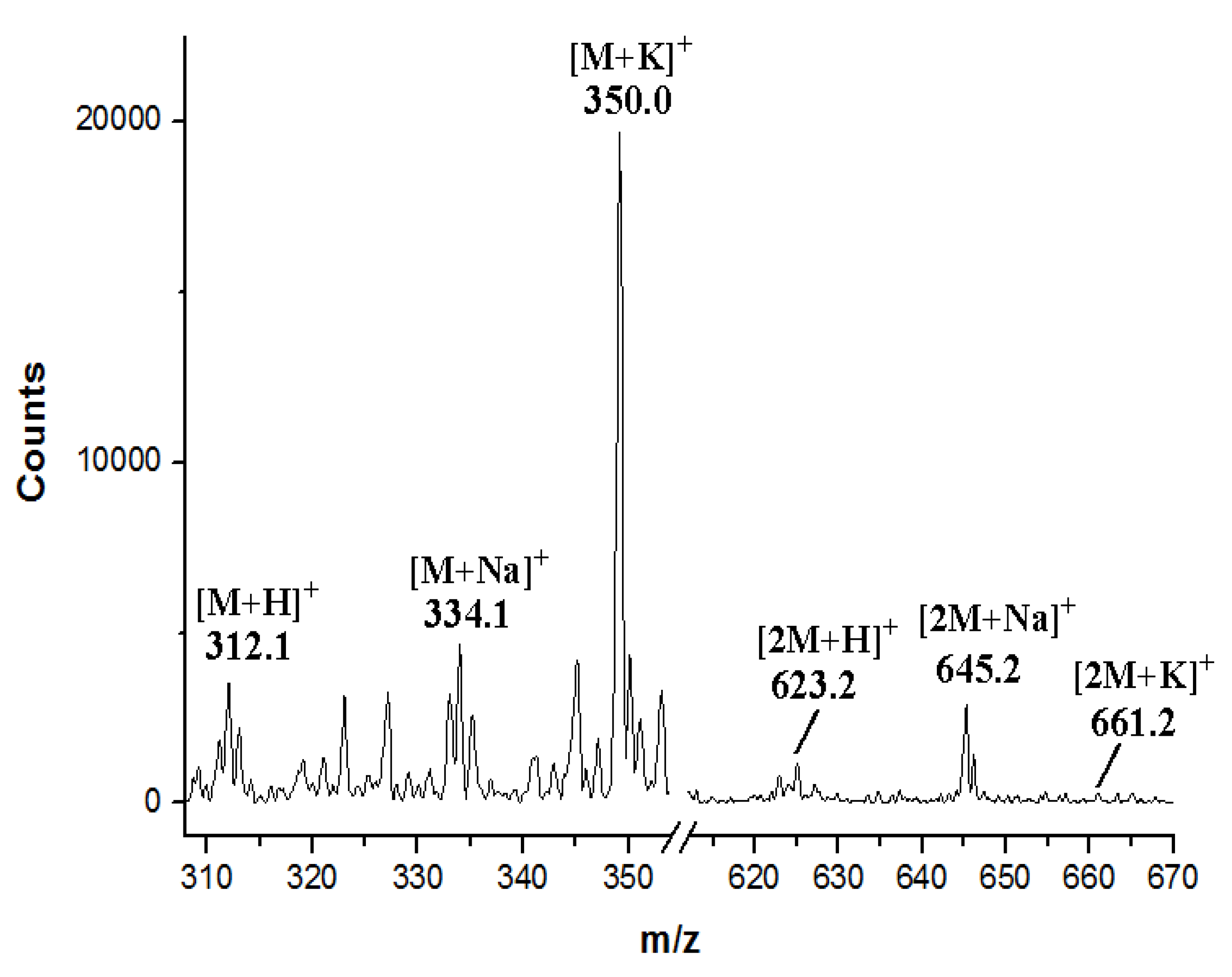
3.7. Production and Analyses of ICZs in S. sanyensis FMA Strains
3.8. Nucleotide Sequence Accession Number
4. Conclusions
Supplementary Material
| Strains or plasmids | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli Top10 | Host strain of cosmid vector SuperCos1 | Invitrogen |
| E. coli DH5a | Host strain for general cloning | Stratagene |
| E. coli ET12567/pUZ8002 | Host strain for conjugation | [39] |
| E. coli BW25113/pIJ790 | Host strain for PCR-targeting | [40] |
| Strptomyces sanyensis FMA | Wild type, ICZs producer | [4] |
| LIW601 | Δ spcC inactivation mutant of S. sanyensis FMA | This study |
| LIW602 | Δ spcI inactivation mutant of S. sanyensis FMA | This study |
| LIW603 | Δ spcR inactivation mutant of S. sanyensis FMA | This study |
| Strptomyces coelicolor M1152/pWLI617 | Strptomyces coelicolor M1152 carrying pWLI617 | This study |
| Plasmids | ||
| SuperCosI | Apr, Kmr, cosmid vector | Stratagene |
| pUM-T | Apr, general cloning vector | Bioteke |
| pIJ773 | Aprr, source of acc(3)IV-oriT cassette | [34] |
| pIJ790 | Cmr, λ RED recombination plasmid | [34] |
| pWLI601 | pUM-T cloned with the amplified FAD-dependent monooxygenase gene fragment | This study |
| pWLI611-615 | Genomic library cosmids harboring spc biosynthetic genes from S. sanyensis FMA | This study |
| pWLI621 | pWLI615 derivative where spcC was replaced with acc(3)IV-oriT cassette | This study |
| pWLI622 | pWLI615 derivative where spcI was replaced with acc(3)IV-oriT cassette | This study |
| pWLI623 | pWLI615 derivative where spcR was replaced with acc(3)IV-oriT cassette | This study |
| pWLI617 | pWLI615 derivative which was equipped with oriT and φC31 attP/int | This study |
| pSET152AB | pSET152 derivative | [41] |
| Gene | Primer pairs used for inactivation (5'→3') |
|---|---|
| spcC | spcCMF: GCCGCCCGACATCCTGGTGGACGGGCCCGAGGGCGACCGGattccggggatccgtcgacc |
| spcCMR: CGCTCGTACAGCTTGGCGACCTCCTCGCCGCCCCCGCCGCGtgtaggctggagctgcttc | |
| spcI | spcIMF: CATGACCACGCGAGTATGGGACTACCTGGCGGAGTACGAGattccggggatccgtcgacc |
| spcIMR: CCTCACAGCGTCTCCAGCACCTCGCGCAGCGCGTGGACGACtgtaggctggagctgcttc | |
| spcR | spcRMF: CATGGGTCCTCAGGTACGAGCGTTAGCACCGCTGCGCGGCattccggggatccgtcgacc |
| spcRMR: CCTCAGGTCCGGAAGCGCACCAGAGCGGTGCGGGAGCGTATtgtaggctggagctgcttc |
| Gene | Primer pairs designed to verify the mutant strains (5'→3') | Length of fragment replaced | Length of desired PCR fragments | |
|---|---|---|---|---|
| Wild type | Mutant | |||
| spcC | spcCCF: AGTTGCCGCCCGACATCC | 321 bp | 409 bp | 1470 bp |
| spcCCR: GCCCGCTCGTACAGCTTGG | ||||
| spcI | spcICF: GACCACGCGAGTATGGGACTAC | 1032 bp | 1111 bp | 1467 bp |
| spcICR: GCCTCACAGCGTCTCCAGCA | ||||
| spcR | spcRCF: GGTCCCGTCCCCTTCGACA | 2883 bp | 3001 bp | 1540 bp |
| spcRCR: GGCCTCAGGTCCGGAAGC | ||||
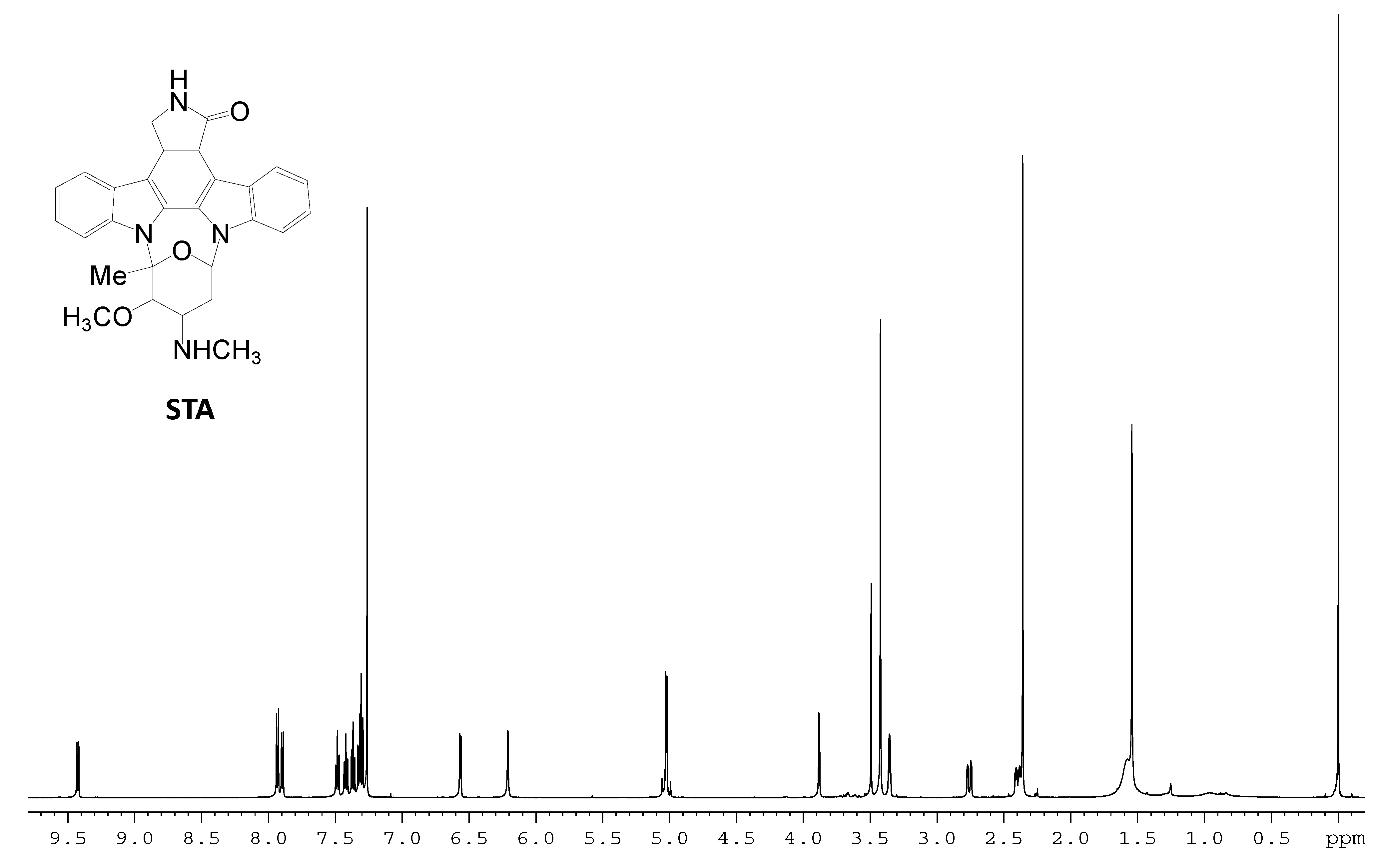
| Position | δH (numbers of H, multiplicity b, J, Hz) |
|---|---|
| 1 | 7.30 (1H, d, 7.9) |
| 2 | 7.48 (1H, dt, 7.7, 1.1) |
| 3 | 7.36 (1H, dt, 7.5, 0.5) |
| 4 | 9.41 (1H, d, 7.9) |
| 6 | 6.21 (1H, brs) |
| 7 | 5.03 (2H, AB, 16.1) |
| 8 | 7.90 (1H, d, 7.7) |
| 9 | 7.32 (1H, t, 7.5) |
| 10 | 7.42 (1H, dt, 7.7,1.1) |
| 11 | 7.93 (1H, d, 8.4) |
| 3′ | 3.88 (1H, d,3.6) |
| 4′ | 3.35 (1H, m) |
| 5′ | 2.41 (1H, ddd, 14.9,5.7,3.7), 2.75 (1H, ddd, 14.9,4.0,1.2) |
| 6′ | 6.57(1H, dd, 5.7,1.1) |
| 2′-CH3 | 2.36 (1H, s) |
| 3′-OCH3 | 3.41 (1H, s) |
| 4′-NCH3 | 1.54 (1H, s) |
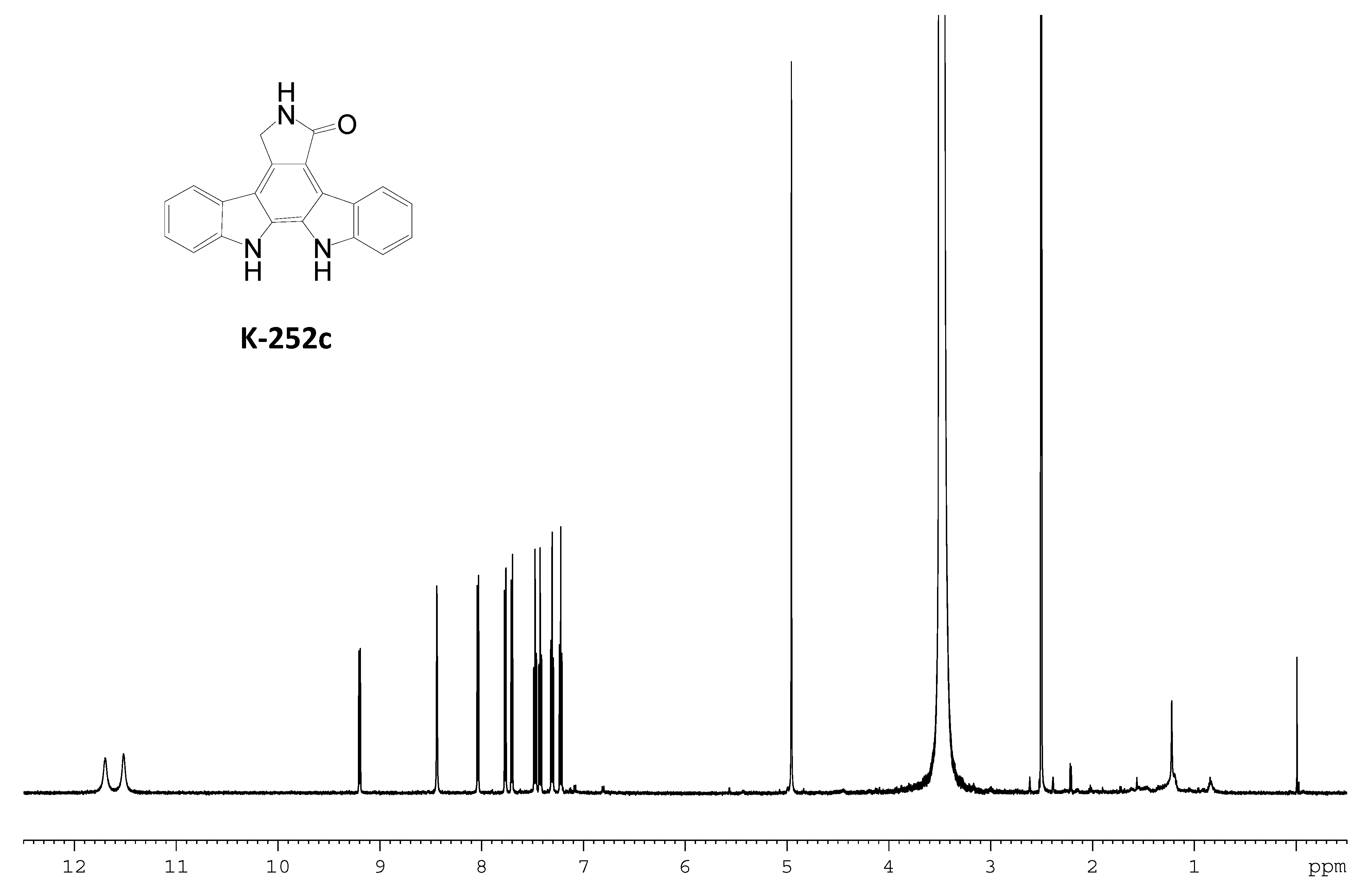
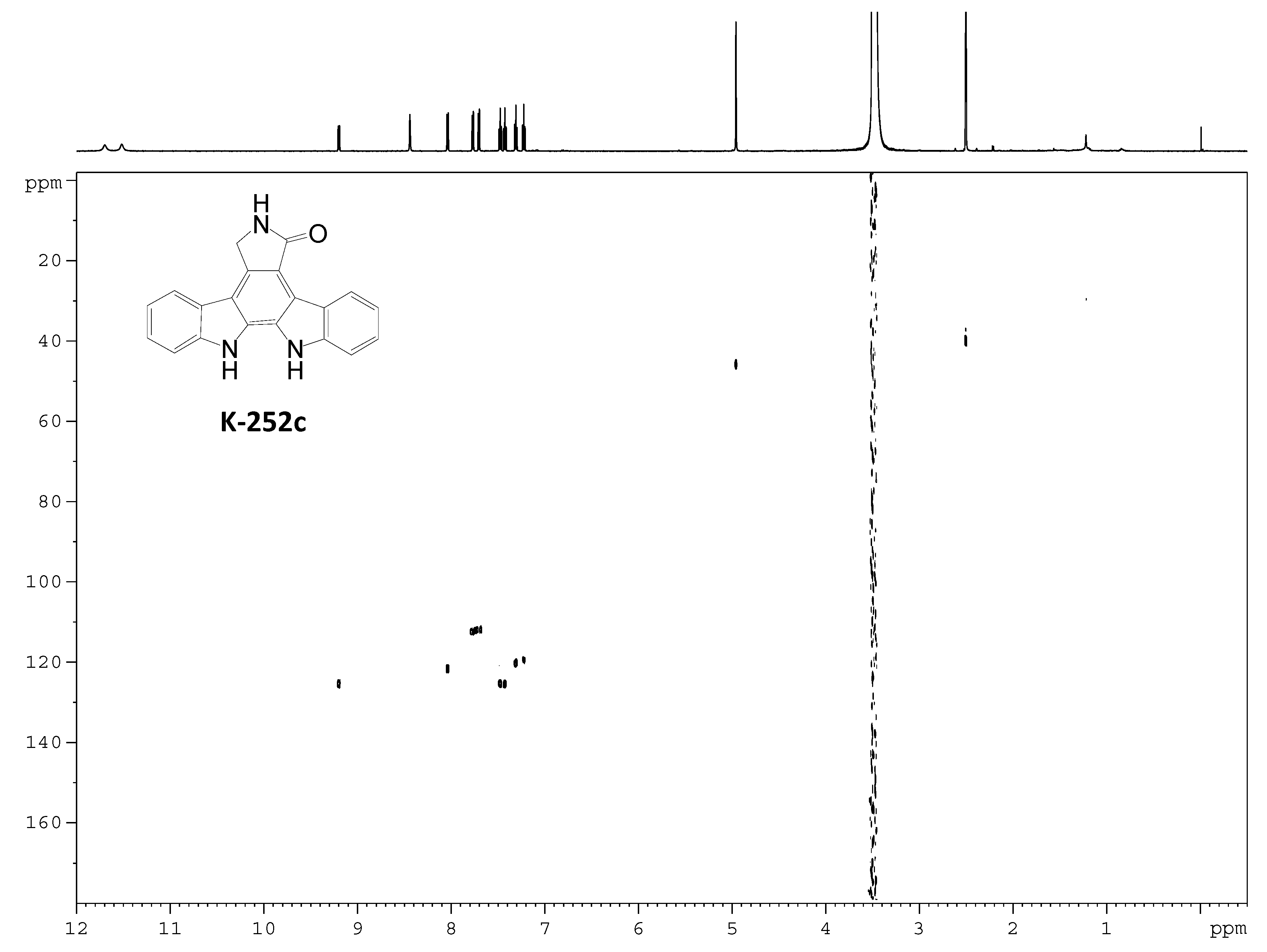
| Position | δH(multiplicity b, J, Hz) | δC |
|---|---|---|
| 1 | 7.70 (d, 8.1) | 111.9 |
| 2 | 7.43 (dt, 7.7, 1.1) | 125.5 |
| 3 | 7.22 (dt, 7.5, 0.8) | 119.4 |
| 4 | 9.20(d, 7.9) | 125.3 |
| 6 | 8.44 (brs) | / |
| 7 | 4.96 (s) | 45.7 |
| 8 | 8.04 (d, 7.7) | 121.6 |
| 9 | 7.31 (dt, 7.5, 0.8) | 120.2 |
| 10 | 7.48 (dt, 7.7,1.1) | 125.3 |
| 11 | 7.77 (d, 8.1) | 112.2 |
| 12 | 11.70(brs) | / |
| 13 | 11.52(brs) | / |
| Position | δH(multiplicity b, J, Hz) | δC |
|---|---|---|
| 1 | 7.69 (d, 8.5) | 110.3 |
| 2 | 7.48 (m) | 125.4 |
| 3 | 7.27 (dt, 7.5, 0.8) | 119.7 |
| 4 | 9.45(d, 7.8) | 125.8 |
| 4a | / | 122.9 |
| 4c | / | 118.8 |
| 5 | / | 172.4 |
| 6 | 8.53 (brs) | / |
| 7 | 5.00 (AB,17.5) | 45.6 |
| 7a | / | 134.4 |
| 7b | / | 115.4 |
| 7c | / | 122.5 |
| 8 | 8.06 (d, 7.8) | 121.5 |
| 9 | 7.31 (dt, 7.4, 0.9) | 120.2 |
| 10 | 7.50 (m) | 125.4 |
| 11 | 7.60 (d, 8.1) | 111.8 |
| 11a | / | 139.4 |
| 12 | 11.68 (brs) | / |
| 12b | / | 124.9 |
| 13a | / | 140.5 |
| 1′ | 6.39 (d, 9.6) | 77.3 |
| 2′ | 4.48 (m) | 67.2 |
| 3′ | 4.17 (dd, 3.6, 5.9) | 72.1 |
| 4′ | 4.04 (dd, 1.0, 3.6) | 71.9 |
| 5′ | 4.47 (m) | 76.7 |
| 6′ | 1.69 (d, 7.3) | 15.8 |



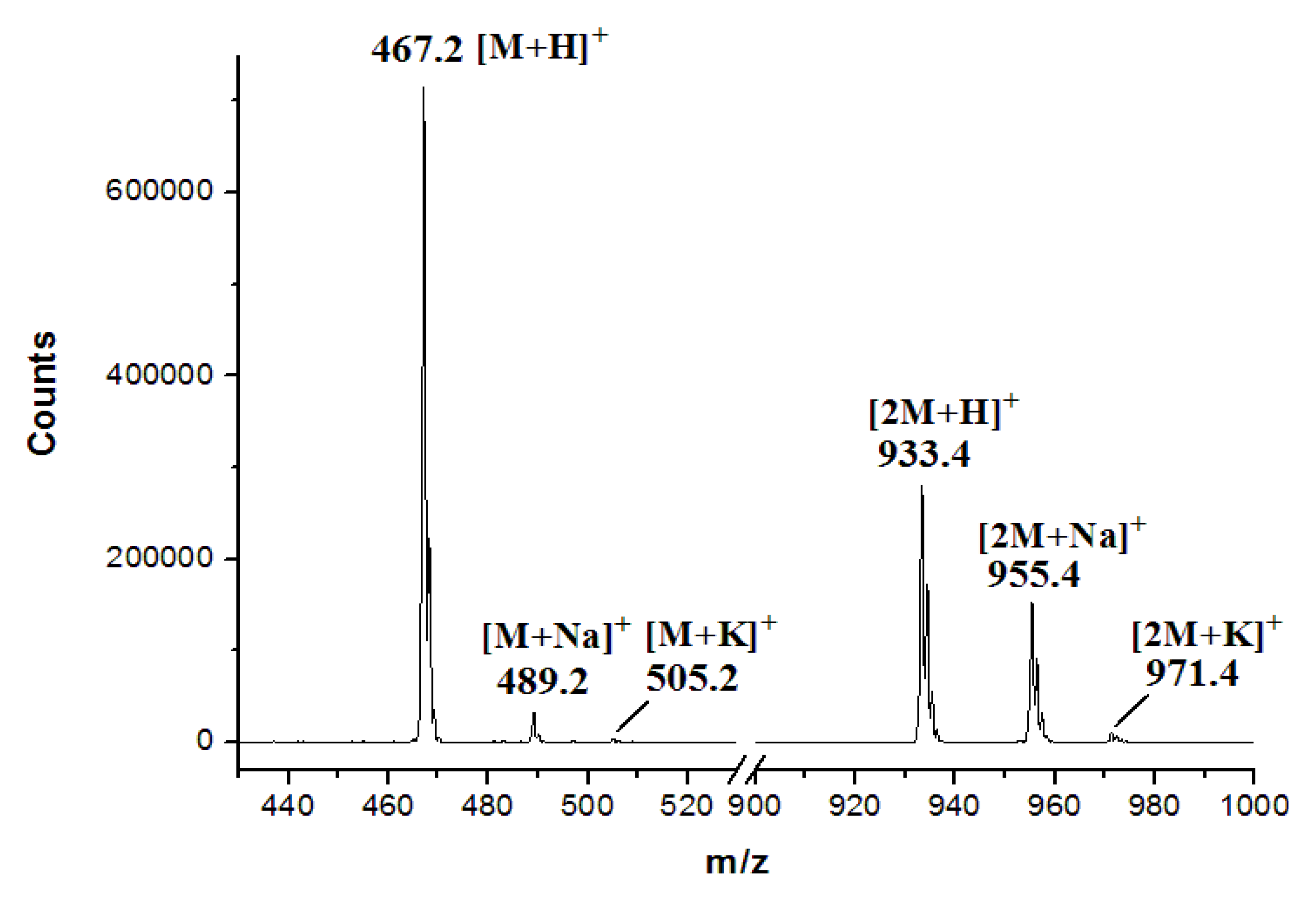




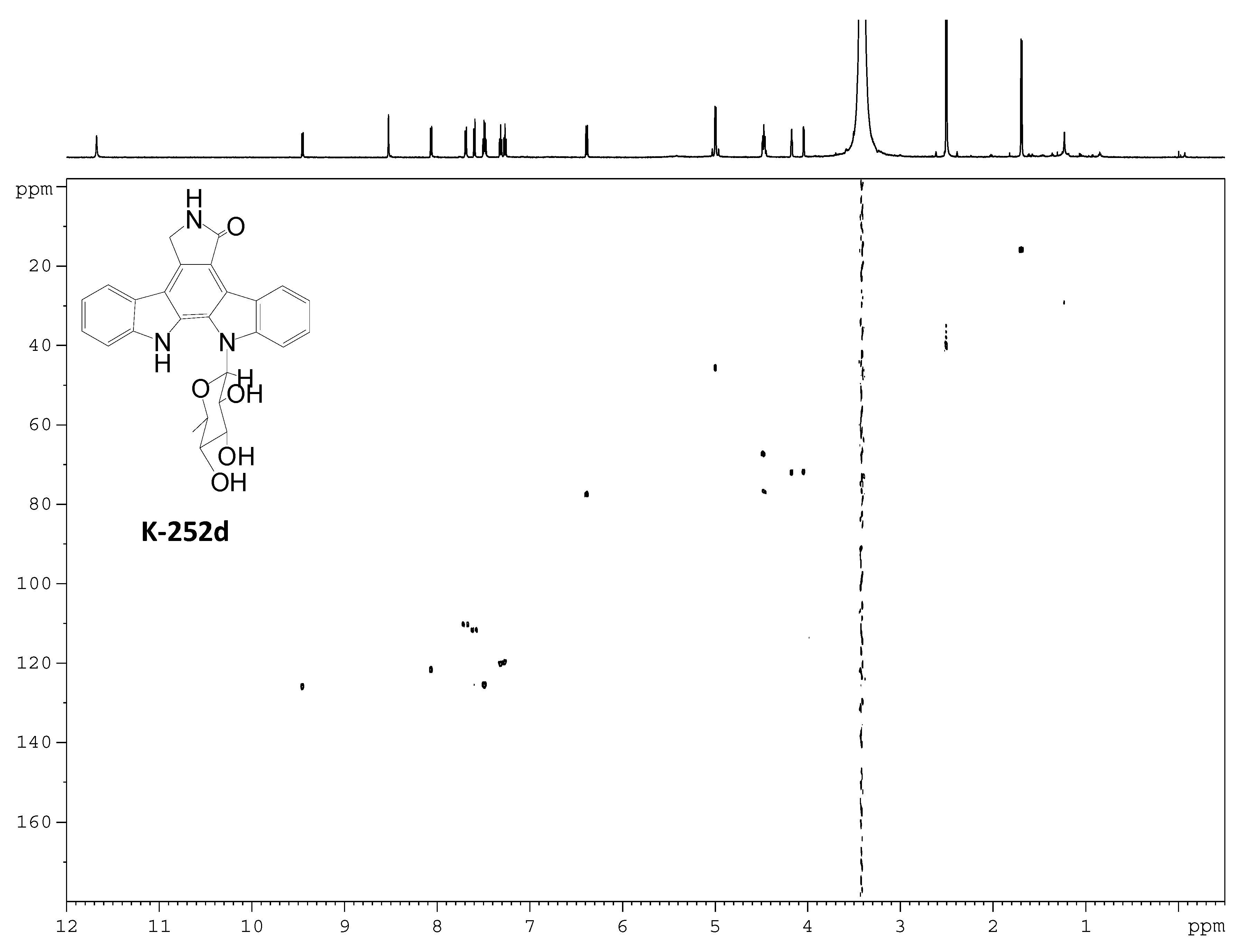
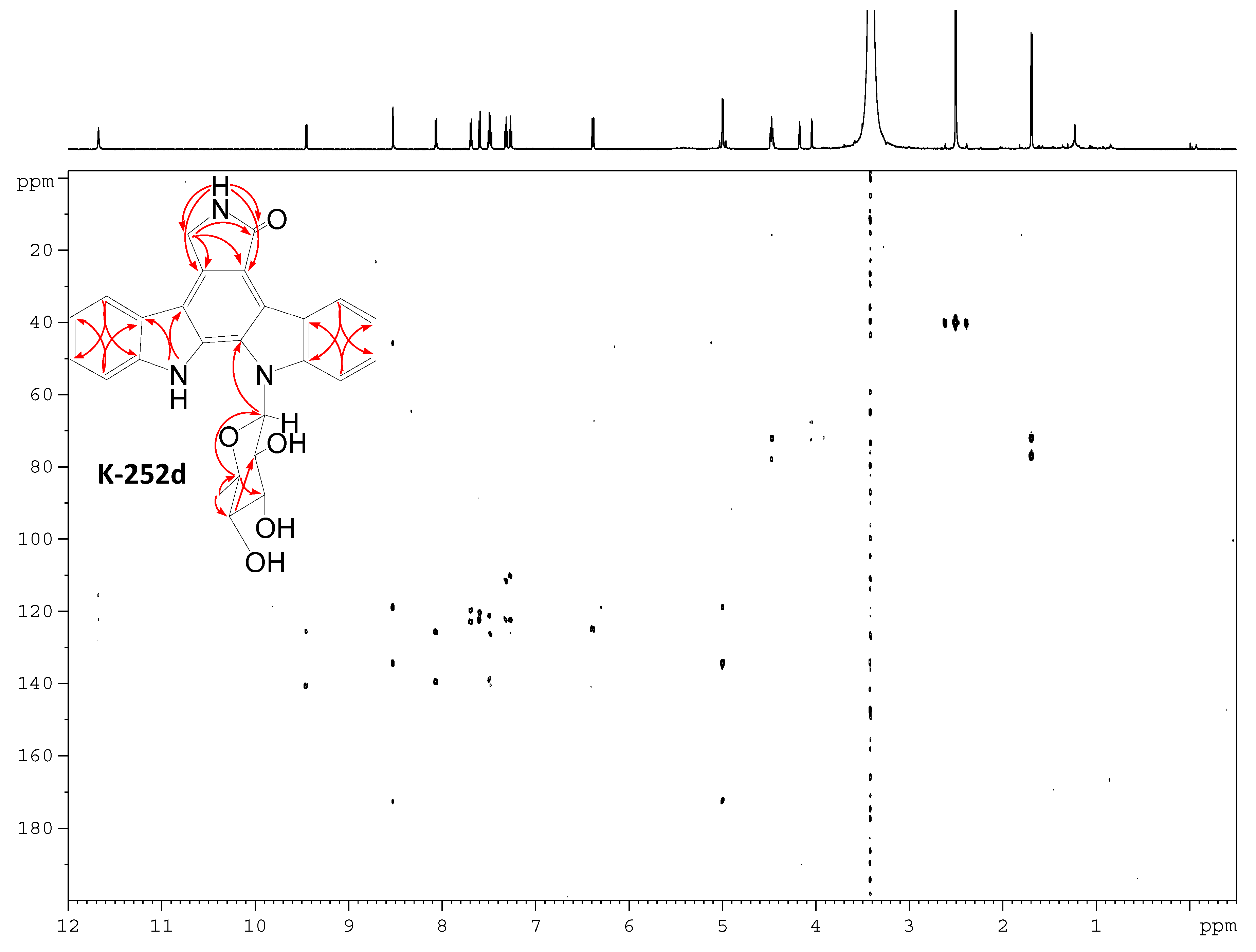
Acknowledgments
References
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchya, H.; Takahashi, Y.; Masuma, R. A new alkaloid AM-2282 of Streptomyces. origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. (Tokyo) 1977, 30, 275–282. [Google Scholar] [CrossRef]
- Sanchez, C.; Mendez, C.; Salas, J.A. Indolocarbazole natural products: Occurrence, biosynthesis, and biological activity. Nat. Prod. Rep. 2006, 23, 1007–1045. [Google Scholar] [CrossRef]
- Reyes, F.; Fernandez, R.; Rodriguez, A.; Bueno, S.; de Eguilior, C.; Francesch, A.; Cuevas, C. Cytotoxic staurosporines from the marine ascidian Cystodytes solitus. J. Nat. Prod. 2008, 71, 1046–1048. [Google Scholar] [CrossRef]
- Fu, P.; Yang, C.; Wang, Y.; Liu, P.; Ma, Y.; Xu, L.; Su, M.; Hong, K.; Zhu, W. Streptocarbazoles A and B, two novel indolocarbazoles from the marine-derived actinomycete strain Streptomyces sp. FMA. Org. Lett. 2012, 14, 2422–2425. [Google Scholar] [CrossRef]
- Acero, N.; Brana, M.F.; Anorbe, L.; Dominguez, G.; Munoz-Mingarro, D.; Mitjans, F.; Piulats, J. Synthesis and biological evaluation of novel indolocarbazoles with anti-angiogenic activity. Eur. J. Med. Chem. 2012, 48, 108–113. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Waller, D.L.; Wang, J.; Liu, Q. Natural products as kinase inhibitors. Nat. Prod. Rep. 2012, 29, 392–403. [Google Scholar] [CrossRef]
- Prudhomme, M. Biological targets of antitumor indolocarbazoles bearing a sugar moiety. Curr. Med. Chem. Anticancer Agents 2004, 4, 509–521. [Google Scholar] [CrossRef]
- Kurosu, M.; Begari, E. Bacterial protein kinase inhibitors. Drug Dev. Res. 2010, 71, 168–187. [Google Scholar]
- Gani, O.A.; Engh, R.A. Protein kinase inhibition of clinically important staurosporine analogues. Nat. Prod. Rep. 2010, 27, 489–498. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: http://www.clinicaltrials.gov/ (accessed on 21 November 2012).
- Onaka, H.; Taniguchi, S.; Igarashi, Y.; Furumai, T. Cloning of the staurosporine biosynthetic gene cluster from Streptomyces sp. TP-A0274 and its heterologous expression in Streptomyces lividans. J. Antibiot. (Tokyo) 2002, 55, 1063–1071. [Google Scholar] [CrossRef]
- Sanchez, C.; Butovich, I.A.; Brana, A.F.; Rohr, J.; Mendez, C.; Salas, J.A. The biosynthetic gene cluster for the antitumor rebeccamycin: Characterization and generation of indolocarbazole derivatives. Chem. Biol. 2002, 9, 519–531. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.S.; Chae, C.S.; Hyun, C.G.; Choi, B.W.; Shin, J.; Oh, K.B. Genetic organization of the biosynthetic gene cluster for the indolocarbazole K-252a in Nonomuraea longicatena JCM 11136. Appl. Microbiol. Biotechnol. 2007, 75, 1119–1126. [Google Scholar] [CrossRef]
- Chiu, H.T.; Chen, Y.L.; Chen, C.Y.; Jin, C.; Lee, M.N.; Lin, Y.C. Molecular cloning, sequence analysis and functional characterization of the gene cluster for biosynthesis of K-252a and its analogs. Mol. Biosyst. 2009, 5, 1180–1191. [Google Scholar]
- Gao, Q.; Zhang, C.; Blanchard, S.; Thorson, J.S. Deciphering indolocarbazole and enediyne aminodideoxypentose biosynthesis through comparative genomics: Insights from the AT2433 biosynthetic locus. Chem. Biol. 2006, 13, 733–743. [Google Scholar] [CrossRef]
- Howard-Jones, A.R.; Walsh, C.T. Enzymatic generation of the chromopyrrolic acid scaffold of rebeccamycin by the tandem action of RebO and RebD. Biochemistry 2005, 44, 15652–15663. [Google Scholar] [CrossRef]
- Onaka, H. Biosynthesis of indolocarbazole and goadsporin, two different heterocyclic antibiotics produced by actinomycetes. Biosci. Biotechnol. Biochem. 2009, 73, 2149–2155. [Google Scholar] [CrossRef]
- Asamizu, S.; Shiro, Y.; Igarashi, Y.; Nagano, S.; Onaka, H. Characterization and functional modification of StaC and RebC, which are involved in the pyrrole oxidation of indolocarbazole biosynthesis. Biosci. Biotechnol. Biochem. 2011, 75, 2184–2193. [Google Scholar] [CrossRef]
- Goldman, P.J.; Ryan, K.S.; Hamill, M.J.; Howard-Jones, A.R.; Walsh, C.T.; Elliott, S.J.; Drennan, C.L. An unusual role for a mobile flavin in StaC-like indolocarbazole biosynthetic enzymes. Chem. Biol. 2012, 19, 855–865. [Google Scholar] [CrossRef]
- Groom, K.; Bhattacharya, A.; Zechel, D.L. Rebeccamycin and staurosporine biosynthesis: Insight into the mechanisms of the flavin-dependent monooxygenases RebC and StaC. ChemBioChem 2011, 12, 396–400. [Google Scholar] [CrossRef]
- Ryan, K.S.; Drennan, C.L. Divergent pathways in the biosynthesis of bisindole natural products. Chem. Biol. 2009, 16, 351–364. [Google Scholar] [CrossRef]
- Sanchez, C.; Zhu, L.; Brana, A.F.; Salas, A.P.; Rohr, J.; Mendez, C.; Salas, J.A. Combinatorial biosynthesis of antitumor indolocarbazole compounds. Proc. Natl. Acad. Sci. USA 2005, 102, 461–466. [Google Scholar]
- Howard-Jones, A.R.; Walsh, C.T. Staurosporine and rebeccamycin aglycones are assembled by the oxidative action of StaP, StaC, and RebC on chromopyrrolic acid. J. Am. Chem. Soc. 2006, 128, 12289–12298. [Google Scholar] [CrossRef]
- Makino, M.; Sugimoto, H.; Shiro, Y.; Asamizu, S.; Onaka, H.; Nagano, S. Crystal structures and catalytic mechanism of cytochrome P450 StaP that produces the indolocarbazole skeleton. Proc. Natl. Acad. Sci. USA 2007, 104, 11591–11596. [Google Scholar] [CrossRef]
- Olano, C.; Mendez, C.; Salas, J.A. Antitumor compounds from marine actinomycetes. Mar. Drugs 2009, 7, 210–248. [Google Scholar]
- Hernandez, L.M.; Blanco, J.A.; Baz, J.P.; Puentes, J.L.; Millan, F.R.; Vazquez, F.E.; Fernandez-Chimeno, R.I.; Gravalos, D.G. 4′-N-methyl-5′-hydroxystaurosporine and 5′-hydroxystaurosporine, new indolocarbazole alkaloids from a marine Micromonospora sp. strain. J. Antibiot. (Tokyo) 2000, 53, 895–902. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, T.; Li, D.; Gu, J.; Xia, W.; Fang, Y.; Liu, H.; Zhu, W.; Gu, Q. Two indolocarbazole alkaloids with apoptosis activity from a marine-derived actinomycete Z(2)039-2. Arch. Pharm. Res. 2007, 30, 270–274. [Google Scholar]
- Sui, J.L.; Xu, X.X.; Qu, Z.; Wang, H.L.; Lin, H.P.; Xie, Q.Y.; Ruan, J.S.; Hong, K. Streptomyces sanyensis sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2010, 61, 1632–1637. [Google Scholar]
- Kui, H. Wuhan University: Wuhan, China, Unpublished work. 2012.
- Meksuriyen, D.; Cordell, G.A. Biosynthesis of staurosporine, 1. 1H- and 13C-NMR assignments. J. Nat. Prod. 1988, 51, 884–892. [Google Scholar] [CrossRef]
- Onaka, H. Biosynthesis of heterocyclic antibiotics in actinomycetes and an approach to synthesize the natural compounds. Actinomycetologica 2006, 20, 62–71. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Paget, M.S.; Chamberlin, L.; Atrih, A.; Foster, S.J.; Buttner, M.J. Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 1999, 181, 204–211. [Google Scholar]
- Gust, B.; Challis, G.L.; Fowler, K.; Kieser, T.; Chater, K.F. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 2003, 100, 1541–1546. [Google Scholar] [CrossRef]
- Gust, B.; Chandra, G.; Jakimowicz, D.; Yuqing, T.; Bruton, C.J.; Chater, K.F. Lambda red-mediated genetic manipulation of antibiotic-producing Streptomyces. Adv. Appl. Microbiol. 2004, 54, 107–128. [Google Scholar] [CrossRef]
- Kieser, T.; Bib, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; The John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Ishikawa, J.; Hotta, K. FramePlot: A new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol. Lett. 1999, 174, 251–253. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- MacNeil, D.J.; Gewain, K.M.; Ruby, C.L.; Dezeny, G.; Gibbons, P.H.; MacNeil, T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 1992, 111, 61–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Chen, Q.; Luo, M.; Sun, A.; Song, Y.; Ma, J.; Ju, J. South Sea Institute of Oceanology, Chinese Academy of Sciences: Guangzhou, China, Unpublished work. 2013.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, T.; Du, Y.; Cui, Q.; Zhang, J.; Zhu, W.; Hong, K.; Li, W. Cloning, Characterization and Heterologous Expression of the Indolocarbazole Biosynthetic Gene Cluster from Marine-Derived Streptomyces sanyensis FMA. Mar. Drugs 2013, 11, 466-488. https://doi.org/10.3390/md11020466
Li T, Du Y, Cui Q, Zhang J, Zhu W, Hong K, Li W. Cloning, Characterization and Heterologous Expression of the Indolocarbazole Biosynthetic Gene Cluster from Marine-Derived Streptomyces sanyensis FMA. Marine Drugs. 2013; 11(2):466-488. https://doi.org/10.3390/md11020466
Chicago/Turabian StyleLi, Tong, Yuanyuan Du, Qiu Cui, Jingtao Zhang, Weiming Zhu, Kui Hong, and Wenli Li. 2013. "Cloning, Characterization and Heterologous Expression of the Indolocarbazole Biosynthetic Gene Cluster from Marine-Derived Streptomyces sanyensis FMA" Marine Drugs 11, no. 2: 466-488. https://doi.org/10.3390/md11020466





