Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae
Abstract
:1. Introduction
| Microalgae/Cyanobacteria | Applications | Main Effects/Type of Action | References |
|---|---|---|---|
| Arthrospira/Spirulina | human nutrition/health food; liquid CO2 extracts (capsules); potential therapeutic | prebiotic food; antioxidant (extract); anti-allergic, anti-inflammatory (extract) | [7,8,9] |
| Chlorella vulgaris | human nutrition/health food and drink supplement; biofertilizer; effluent treatment | growth factor (drink) | [7,10,11,12] |
| Chlorella stigmatophora; Phaeodactylum tricornutum | potential therapeutic (hydrosoluble extract) | anti-inflammatory, analgesic, free radical scavenging | [13] |
| Tetraselmis; Pavlova lutheri | food for bivalves/shellfish | [7] | |
| larvae (aquaculture) | |||
| Isochrysis; Pleurochrysis carterae | food for bivalves/shellfish larvae (aquaculture); potential therapeutic | anti-allergic, anti-inflammatory (extract) | [7,8] |
| Nannochloropsis | feed in aquaculture | [7] | |
| Dunaliella | human nutrition (powder); oil extracts with carotenoids (capsules); potential therapeutic | anti-allergic, anti-inflammatory (extract) | [7,8,14,15] |
| Odontella aurita | human nutrition | [7] | |
| Porphyridium purpureum; Rhodosorus marinus | potential therapeutic | anti-allergic, anti-inflammatory (extract) | [8] |
2. The Polysaccharides from Marine Microalgae: from the Sources to the Applications
| Microalgae/Cyanobacteria | Group | Type of Polysaccharide | Main Sugars | References |
|---|---|---|---|---|
| Cylindrotheca closterium | diatoms | sPS | xylose, glucose | [22,23] |
| Navicula salinarum | sPS | glucose, xylose | ||
| Phaeodactylum tricornutum | EPS (sulphated) | glucose, mannose | [24,25] | |
| Haslea ostrearia | EPS | [26] | ||
| Nitzschia closterium | EPS | [27] | ||
| Skeletonema costatum | EPS | |||
| Chaetoceros sp. | EPS | |||
| Amphora sp. | EPS | [25] | ||
| Chlorella stigmatophora | chlorophytes | PS (sulphated) | glucose, xylose | [28] |
| Chlorella sp. | sPS | [25,29,30] | ||
| C. autotrophica | sPS | |||
| Ankistrodesmus angustus | EPS | |||
| Tetraselmis sp. | prasinophyte (Chlorophyta) | sPS | ||
| Isochrysis sp. | prymnesiophyte/haptophyte | sPS | ||
| Porphyridium sp. | rhodophytes | sPS | xylose, galactose | [31,32,33] |
| P. cruentum | sPS | xylose, galactose | [30,34,35] | |
| P. purpureum | sPS | [36] | ||
| Rhodella reticulata | sPS | xylose, galactose | [31,32] | |
| Cochlodinium polykrikoides | dinoflagellates | sPS | mannose, galactose | [37] |
| Gyrodinium impudicum | sPS | galactose | [38] | |
| Aphanothece halophytica | cyanophytes | EPS | glucose, fucose | [39] |
| Arthrospira platensis | sPS and intracellular sulphated calcium spirulan (CaSp) | rhamnose, fructose | [36,40,41] | |
| Anabaena, Aphanocapsa, Cyanothece, Gloethece, Nostoc, Phormidium, Synechocystis | sPS | [42] | ||
2.1. Marine Unicellular Algae Producing EPS
| Microalgae/Cyanobacteria | Group | Virus strain | Family/Group of virus | Cell-Lines | EC50/ED50 (μg/mL) | References |
|---|---|---|---|---|---|---|
| A. platensis; A. maxima | cyanobacteria | vaccinia virus VACV and VACV-GFP; ectromelia virus (ECTV); HSV-1, HSV-2, human cytomegalovirus (HCMV), measles virus, mumps virus, HIV-1, Flu-A | Orthopoxvirus/Poxviridae; Simplexvirus/Herpesviridae; Herpesviridae; Morbillivirus/Paramyxoviridae; Rubulavirus/Paramyxoviridae; Lentivirus/Retroviridae; Influenzavirus/Orthomyxoviridae | HEp-2 and Vero C1008; HeLa, HEL, Vero, MDCK | 0.78; 69; 0.92–16.5; 8.3–41; 17–39; 23–92; 2.3–11.4; 9.4–230 | [36,40,43,44] |
| Porphyridium sp. | rhodophytes | herpes simplex virus HSV-1 and HSV-2; varicela zoster virus (VZV); murine sarcoma virus (MuSV-124) and MuSV/MuLV (murine leukemia virus) | Simplexvirus/Herpesviridae; Varicellovirus/Herpesviridae; Gammaretrovirus/Retroviridae (type VI) | NIH/3T3 | 1–5 (in vivo, 100); 0.7; 10 and 5 (RT50) | [45,46,47] |
| P. cruentum | hepatitis B virus (HBV); viral haemorrhagic septicaemia virus (VHSV); African swine fever virus (ASFV); vaccinia virus (VACV); vesicular stomatitis virus (VSV) | Orthohepadnavirus/Hepadnaviridae; Novirhabdovirus/Rhabdoviridae; Asfarvirus/Asfarviridae; Orthopoxvirus/Poxviridae; Vesiculovirus/Rhabdoviridae | HEL | 20, 200 (exocellular extracts); 12–56; 20–45 | [48,49,50,51] | |
| P. purpureum | vaccinia virus VACV and VACV-GFP; ectromelia virus (ECTV) | Orthopoxvirus/Poxviridae | HEp-2, Vero C1008 | 0.65 | [36] | |
| R. reticulata | herpes simplex virus HSV-1 and HSV-2; varicela zoster virus (VZV); murine sarcoma virus (MuSV-124) and MuSV/MuLV (murine leukemia virus) | Simplexvirus/Herpesviridae; Varicellovirus/Herpesviridae; Gammaretrovirus/Retroviridae (type VI) | NIH/3T3 | 10–20; 8; 150 and 50 (RT50) | [46,47] | |
| G. impudicum | dinoflagellates | Encephalomyocarditis virus; influenza A virus (Flu-A) | Cardiovirus/Picornaviridae; Orthomyxoviridae | MDCK | 0.19–0.48 | [52] |
| C. polykrikoides | Flu-A and Flu-B; respiratory syncytial virus type A (RSV-A) and B (RSV-B); HIV-1; HSV-1; parainfluenza virus type 2 (PFluV-2) | Orthomyxoviridae; Pneumovirus/Paramyxoviridae; Retroviridae; Herpesviridae; Rubulavirus/Paramyxoviridae | MDCK, Hep-2, MT-4, HMV-2 | 0.45–1.1 and 7.1–8.3; 2.0–3.0 and 0.8; 1.7; 4.52–21.6; 0.8–25.3 | [37] |
| Microalgae/Cyanobacteria | Applications | Cells/Animals used for in vitro/in vivo studies | References |
|---|---|---|---|
| Porphyridium | health foods, nutraceutical and functional foods | rats | [53,54] |
| Rhodella; Porphyridium | antioxidant and free radical scavenging | 3T3; mouse liver homogenates and erythrocytes haemolysates, sarcoma 180 cells/mice | [55,56,57,58] |
| Porphyridium, P. cruentum; R. reticulata | anti-lipidaemic, antiglycaemic | rats/mice, chickens | [54,59,60,61] |
| Porphyridium; Chlorella stigmatophora, Phaeodactylum tricornutum | anti-inflammatory and immunomodulatory | polymorphonuclear leukocytes/human dermal microvascular endothelial cells, humans; rabbits and sheep (bone joints); mice macrophages/mice and rats | [28,55,62,63] |
| Porphyridium, R. reticulata; Gyrodinium impudicum; A. platensis | prevention of tumour cell growth | FD early myeloid cell line, 24-1 and EL-4 T-lymphoma cell lines; Graff myeloid cells; rats | [42,64,65,66] |
| Phaeodactylum, Tetraselmis | anti-adhesive | HeLa S3/sand bass culture cells | [30,67] |
| Porphyridium | biolubricant (for bone joints) | [63,68] | |
| Porphyridium, R. reticulata | ion exchanger | [69] | |
| P. cruentum, R. reticulata; R. maculata | drag-reducers | [70,71] | |
2.2. Structure and Rheology of the Exopolysaccharides
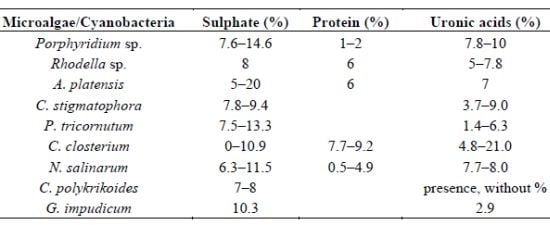
| Microalgae/Cyanobacteria | Sulphate (%) | Protein (%) | Uronic acids (%) | References |
|---|---|---|---|---|
| Porphyridium sp. | 7.6–14.6 | 1–2 | 7.8–10 | [31,55,80] |
| Rhodella sp. | 8 | 6 | 5–7.8 | [31,81] |
| A. platensis | 5–20 | 6 | 7 | [82] |
| C. stigmatophora | 7.8–9.4 | 3.7–9.0 | [28] | |
| P. tricornutum | 7.5–13.3 | 1.4–6.3 | [28] | |
| C. closterium | 0–10.9 | 7.7–9.2 | 4.8–21.0 | [22] |
| N. salinarum | 6.3–11.5 | 0.5–4.9 | 7.7–8.0 | [22] |
| C. polykrikoides | 7–8 | presence, without % | [37] | |
| G. impudicum | 10.3 | 2.9 | [38] |
2.3. Biological Activities and Applications
2.3.2. Activity as Antioxidants and Free Radical Scavenging
2.3.3. Anti-Inflammatory and Immunomodulatory Activities
2.3.4. Inhibition of Tumour Cell Growth
2.3.5. Hypolipidaemic and Hypoglycaemic Properties
2.3.6. Anti-Adhesive Agents
2.3.7. Anticoagulant and Antithrombotic Activity
2.3.8. Biolubricant Properties
2.3.9. Drag-Reducers
2.3.10. Other Applications
3. Final Remarks
Acknowledgements
References
- Yamagushi, K. Recent advances in microalgal bioscience in Japan, with special reference to utilization of biomass and metabolites: A review. J. Appl. Phycol. 1997, 8, 487–502. [Google Scholar] [CrossRef]
- Liang, S.; Xueming, L.; Chen, F.; Chen, Z. Current microalgal health food R & D activities in China. Hydrobiologia 2004, 512, 45–48. [Google Scholar] [CrossRef]
- Vilchez, C.; Garbayo, I.; Lobato, M.V.; Vega, J.M. Microalgae-Mediated chemicals production and wastes removal. Enzyme Microb. Technol. 1997, 20, 562–572. [Google Scholar] [CrossRef]
- Apt, K.A.; Behrens, P.W. Commercial developments in microalgal biotechnology. J. Phycol. 1999, 35, 215–226. [Google Scholar] [CrossRef]
- Müller-Feuga, A. The role of microalgae in aquaculture: situation and trends. J. Appl. Phycol. 2000, 12, 527–534. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae for aquaculture: Opportunities and constraints. J. Appl. Phycol. 1997, 9, 393–401. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Fujitani, N.; Sakari, S.; Yamagushi, Y.; Takenaka, H. Inhibitory effects of microalgae on activation of hyaluronidase. J. Appl. Phycol. 2001, 13, 489–492. [Google Scholar] [CrossRef]
- Blinkova, L.P.; Gorobets, O.B.; Baturo, A.P. Biological activity of Spirulina (in Russian). Zhur. Mikrobiol. Epidemiol. Immunobiol. 2001, 5, 114–118. [Google Scholar]
- Rasmussen, R.S.; Morrissey, M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Morais, R.M.S.C. Chlorella vulgaris as soil amendment: Influence of encapsulation and enrichment with rhizobacteria. Int. J. Agric. Biol. 2011, 13, 719–724. [Google Scholar]
- Raposo, M.F.J.; Oliveira, S.E.; Castro, P.M.; Bandarra, N.M.; Morais, R.M. On the utilization of microalgae for brewery effluent treatment and possible applications of the produced biomass. J. Inst. Brew. 2010, 116, 285–292. [Google Scholar] [CrossRef]
- Guzman, S.; Gato, A.; Calleja, J.M. Anti-Inflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2001, 15, 224–230. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae as source of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Masjuk, N.P. Morphology, Taxonomy, Ecology, Geographical Distribution and Utilization of Dunaliella (in Russian); Naukowa: Kiev, Ukraine, 1973. [Google Scholar]
- Findlay, J.A.; Patil, S.D. Antibacterial constituents of the diatom Navicula delognei. J. Nat. Prod. 1984, 47, 815–818. [Google Scholar] [CrossRef]
- Villar, R.; Laguna, M.R.; Calleja, J.M.; Cadavid, I. Effects of Skeletonema costatum extracts on the central nervous system. Planta Med. 1992, 58, 398–404. [Google Scholar] [CrossRef]
- Laguna, M.R.; Villar, R.; Calleja, J.M.; Cadavid, I. Effects of Chlorella stigmatophora extracts on the central nervous system. Planta Med. 1993, 59, 125–130. [Google Scholar] [CrossRef]
- Villar, R.; Laguna, M.R.; Cadavid, I.; Calleja, J.M. Effects of aqueous extracts of six marine microalgae on smooth muscle contraction in the rat duodenum and vas deferens. Planta Med. 1994, 60, 521–526. [Google Scholar] [CrossRef]
- Laguna, M.R.; Rodriguez-Linares, B.; Villar, R.; Cadavid, I.; Cano, E. Anti-Platelet effects of water soluble extracts from cultured microalgae. In Ethnopharmacologie: Sources, Methodes, Objectifs: Actes du 1er Colloque Européen d’Ethnopharmacologie; Fleurentin, J., Ed.; ORSTOM Editions: Metz, France, 1991; pp. 426–430. [Google Scholar]
- Yoon, H.S.; Müller, K.M.; Sheath, R.G.; Oh, F.D.; Bhattacharya, D. Defining the major lineages of the red algae (Rhodophyta). J. Phycol. 2006, 42, 482–492. [Google Scholar] [CrossRef]
- Staats, N.; de Winder, B.; Stal, L.J.; Mur, L.R. Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum. Eur. J. Phycol. 1999, 34, 161–169. [Google Scholar] [CrossRef]
- Pletikapic, G.; Radic, T.M.; Zimmermann, A.H.; Svetlicic, V.; Pfannkuchen, M.; Maric, D.; Godrjan, J.; Zutic, V. AFM imaging of extracellular polymer release by marine diatom Cylindrotheca closterium (Ehrenberg) Reiman & JC Lewin. J. Mol. Recogn. 2011, 24, 436–445. [Google Scholar] [CrossRef]
- Guzmán-Murillo, M.A.; López-Bolaños, C.C.; Ledesma-Verdejo, T.; Roldan-Libenson, G.; Cadena-Roa, M.A.; Ascencio, F. Effects of fertilizer-based culture media on the production of exocellular polysaccharides and cellular superoxide dismutase by Phaeodactylum tricornutum (Bohlin). J. Appl. Phycol. 2007, 19, 33–40. [Google Scholar] [CrossRef]
- Chen, C.-S.; Anaya, J.M.; Zhang, S.; Spurgin, J.; Chuang, C.-Y.; Xu, C.; Miao, A.-J.; Chen, E.Y.-T.; Schwehr, K.A.; Jiang, Y.; et al. Effects of engineered nanoparticles on the assembly of exopolymeric substances from phytoplankton. PLoS One 2011, 6, 1–7. [Google Scholar]
- Rincé, Y.; Lebeau, T.; Robert, J.M. Artificial cell-immobilization: A model simulating immobilization in natural environments? J. Appl. Phycol. 1999, 11, 263–272. [Google Scholar] [CrossRef]
- Penna, A.; Berluti, S.; Penna, N.; Magnani, M. Influence of nutrient ratios on the in vitro extracellular polysaccharide production by marine diatoms from Adriatic Sea. J. Plankton Res. 1999, 21, 1681–1690. [Google Scholar] [CrossRef]
- Guzman, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-Inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef]
- Yingying, S.; Changhai, W. The optimal growth conditions for the biomass production of Isochrysis galbana and the effects that phosphorus, Zn2+, CO2, and light intensity have on the biochemical composition of Isochrysis galbana and the activity of extracellular CA. Biotechnol. Bioprocess Eng. 2009, 14, 225–231. [Google Scholar] [CrossRef]
- Guzmán-Murillo, M.A.; Ascencio, F. Anti-Adhesive activity of sulphated exopolysaccharides of microalgae on attachment of the red sore disease-associated bacteria and Helicobacter pylori to tissue culture cells. Lett. Appl. Microbiol. 2000, 30, 473–478. [Google Scholar] [CrossRef]
- Geresh, S.; Arad, S.M. The extracellular polysaccharides of the red microalgae: Chemistry and rheology. Bioresour. Technol. 1991, 38, 195–201. [Google Scholar] [CrossRef]
- Dubinsky, O.; Barak, Z.; Geresh, S.; Arad, S.M. Composition of the cell-wall polysaccharide of the unicellular red alga Rhodella reticulata at two phases of growth. In Recent Advances in Algal Biotechnology, the 5th International Conference of the Society of Applied Algology; Office of Naval Research: Tiberias, Israel, 1990; p. 17. [Google Scholar]
- Arad, S.M. Production of sulphated polysaccharides from red unicellular algae. In Algal Biotechnology; Stadler, T., Mollion, J., Verdus, M.C., Karamanos, Y., Morvan, H., Christiaen, D., Eds.; Elsevier Applied Science: London, UK, 1988; pp. 65–87. [Google Scholar]
- Garcia, D.; Morales, E.; Dominguez, A.; Fábregas, J. Productividad mixotrófica del exopolisacárido sulfatado com la microalga marina Porphyridium cruentum. In Communicaciones del III Congreso Ibérico de Biotecnología—Biotec’96; Universidad de Valladolid: Valladolid, Spain, 1996; pp. 591–592. [Google Scholar]
- Kieras, J.H. Study of the Extracellular Polysaccharide of Porphyridium cruentum.
- Radonic, A.; Thulke, S.; Achenbach, J.; Kurth, A.; Vreemann, A.; König, T.; Walter, C.; Possinger, K.; Nitsche, A. Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to Vaccinia virus. J. Antivir. Antiretrovir. 2010, 2, 51–55. [Google Scholar]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulphated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped virus. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar] [CrossRef]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007, 98, 361–367. [Google Scholar] [CrossRef]
- Li, P.; Liu, Z.; Xu, R. Chemical characterization of the released polysaccharides from the cyanobacterium Aphanothece halophytica GR02. J. Appl. Phycol. 2001, 13, 71–77. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef]
- Martinez, M.J.A.; del Olmo, L.M.B.; Benito, P.B. Antiviral activities of polysaccharides from natural sources. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier B.V.: London, UK, 2005; Volume 30, pp. 393–418. [Google Scholar]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fisher, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, T.; Kojima, I. A natural sulphated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus. AIDS Res. Human Retrovir. 1996, 12, 1463–1471. [Google Scholar] [CrossRef]
- Hernandez-Corona, A.; Nieves, I.; Meckes, M.; Chamorro, G.; Barron, B.L. Antiviral activity of Spirulina maxima against herpes simplex virus type 2. Antivir. Res. 2002, 56, 279–285. [Google Scholar] [CrossRef]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Activity of Porphyridium sp. polysaccharide against Herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200. [Google Scholar] [CrossRef]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Antiviral effect of the red microalgal polysaccharides on Herpes simplex and Varidella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134. [Google Scholar] [CrossRef]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Antiviral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002, 2, 8–14. [Google Scholar]
- Huang, J.; Chen, B.; You, W. Studies on separation of extracellular polysaccharide from Porphyridium cruentum and its anti-HBV activity in vitro (in Chinese). Chin. J. Mar. Drugs 2005, 24, 18–21. [Google Scholar]
- Fabregas, J.; García, D.; Fernandez-Alonso, M.; Rocha, A.I.; Gómez-Puertas, P.; Escribano, J.M.; Otero, A.; Coll, J.M. In vitro inhibition of the replication of viral haemorrhagic septicaemia virus (VHSV) and African swine fever virus (ASFV) by extracts from marine microalgae. Antivir. Res. 1999, 44, 67–73. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Morais, A.M.M.B.; Morais, R.M.S.C. Antibacterial and antiviral activities of the exopolysaccharide from Porphyridium cruentum. Z. Naturforsch. C J. Biosci. 2012. submitted. [Google Scholar]
- Vieira, V.V.; Morais, R.M.S.C. Composições constituídas por polissacarídeos com actividade anti-viral e anti-adesão bacteriana, respectivas formulações, processo de elaboração das mesmas e suas utilizações. Portugal Patent 38122.08, 2008. [Google Scholar]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, C.K.; Rhie, K.T.; Lee, H.K. Antiviral effects of sulphated polysaccharide from the marine microalga Gyrodinium impudicum strain KG03. Mar. Biotechnol. 2004, 6, 17–25. [Google Scholar] [CrossRef]
- Dvir, I.; Chayoth, R.; Sod-Moriah, U.; Shany, S.; Nyska, A.; Stark, A.H.; Madar, Z.; Arad, S.M. Soluble polysaccharide of red microalga Porphyridium sp. alters intestinal morphology and reduces serum cholesterol in rats. Br. J. Nutr. 2000, 84, 469–476. [Google Scholar]
- Dvir, I.; Stark, A.H.; Chayoth, R.; Madar, Z.; Arad, S.M. Hycholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp. in rats. Nutrients 2009, 1, 156–167. [Google Scholar] [CrossRef]
- Sun, L. Preparation of Polysaccharides from Porphyridium cruentum and Their Biological Activities. Available online: http://www.latest-science-articles.com/Engineering_Science/Preparation-of-Polysaccharide-from-Porphyridium-Cruentum-and-Its-Biological-Acti-12122.html (accessed on 6 July 2012). Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2010..
- Chen, B.; You, B.; Huang, J.; Yu, Y.; Chen, W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J. Microbiol. Biotechnol. 2010, 26, 833–840. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; van Moppes, D.; Grossman, S.; Arad, S.M. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Arad, S.M. Polysaccharides of red microalgae. In Chemicals from Microalgae; Cohen, Z., Ed.; Taylor & Francis: London, UK, 1999; pp. 282–291. [Google Scholar]
- Ginzberg, A.; Cohen, M.; Sod-Moriah, U.A.; Shany, S.; Rosenshtrauch, A.; Arad, S.M. Chickens fed with biomass of the red microalga Porphyridium sp. have reduced blood cholesterol levels and modified fatty acids composition in egg yolk. J. Appl. Phycol. 2000, 12, 325–330. [Google Scholar] [CrossRef]
- Huang, J.; Liu, L.; Yu, Y.; Lin, W.; Chen, B.; Li, M. Reduction in the blood glucose level of exopolysaccharide of Porphyridium cruentum in alloxan-induced diabetic mice (in Chinese). J. Fujian Norm. Univ. 2006, 22, 77–80. [Google Scholar]
- Matsui, S.M.; Muizzudin, N.; Arad, S.M.; Marenus, K. Sulfated polysaccharides from red microalgae anti-inflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef]
- Arad, S.M.; Atar, D. Viscosupplementation with algal polysaccharides in the treatment of arthritis. WIPO Patent WO/2007/066340, 14 June 2007. [Google Scholar]
- Geresh, S.; Mamontov, A.; Weinstein, J. Sulfation of extracellular polysaccharides of red microalga: Preparation, characterization, properties. J. Biochem. Biophys. Methods 2002, 50, 179–187. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Minkova, K.; Gigova, L. Cancer protective action of polysaccharide derived from microalga Porphyridium cruentum—A biological background. Biotechnol. Biotechnol. Equip. 2009, 23, 783–787. [Google Scholar]
- Shopen-Katz, O.; Ling, E.; Himelfarb, Y.; Lamprecht, S.A.; Arad, S.M.; Shany, S. The effect of Porphyridium sp., biomass and of its polysaccharide in prevention and inhibition of human colon cancer. In Proceedings of the Era of Biotechnology, Beer-Sheva, Israel, 24–27 October 2000; p. 32.
- Dade, W.B.; Davis, J.D.; Nichols, P.D.; Nowell, A.R.M.; Thistle, D.; Trexler, M.B.; White, D.C. Effects of bacterial exopolymer adhesion on the entrainment of sand. Geomicrobiol. J. 1990, 8, 1–16. [Google Scholar] [CrossRef]
- Arad, S.M.; Rapoport, L.; Moshkovich, A.; van Moppes, D.; Karpasan, M.; Golan, R.; Golan, Y. Superior biolubricant from a species of red microalga. Langmuir 2006, 2, 7313–7317. [Google Scholar]
- Lupescu, N.; Geresh, S.; Arad, S.M.; Bernstein, M.; Glaser, R. Structure of some sulfated sugars isolated after acid hydrolysis of the extracellular polysaccharide of Porphyridium sp. unicellular red alga. Carbohydr. Res. 1991, 210, 349–352. [Google Scholar] [CrossRef]
- Gasljevic, K.; Hall, K.; Chapman, D.; Matthys, E.F. Drag-Reducing polysaccharides from marine microalgae: Species productivity and drag reduction effectiveness. J. Appl. Phycol. 2008, 20, 299–310. [Google Scholar]
- Ramus, J.; Kenney, B.E.; Shaughnessy, E.J. Drag-Reducing properties of microalgal exopolymers. Biotechnol. Bioeng. 1989, 33, 550–557. [Google Scholar]
- Geresh, S.; Adin, I.; Yarmolinsky, E.; Karpasas, M. Characterization of the extracellular polysaccharide of Porphyridium sp.: Molecular weight determination and rheological properties. Carbohydr. Polym. 2002, 50, 183–189. [Google Scholar] [CrossRef]
- You, T.; Barnett, S.M. Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem. Eng. J. 2004, 19, 251–258. [Google Scholar] [CrossRef]
- Geresh, S.; Dubinsky, O.; Arad, S.M.; Christiaen, D.; Glaser, R. Structure of 3-O-(α-D-glucopyranosyluronic acid)-L-galactopyronase, an aldobiuronic acid isolated from polysaccharide of various unicellular red algae. Carbohydr. Res. 1990, 208, 301–305. [Google Scholar] [CrossRef]
- Eteshola, E.; Karpasas, M.; Arad, S.M.; Gottlieb, M. Red microalga exopolysaccharides: 2. Study of the rheology, morphology and thermal gelation of aqueous preparations. Acta Polym. 1998, 49, 549–556. [Google Scholar] [CrossRef]
- Heaney-Kieras, J.; Roden, L.; Chapman, D.J. The covalent linkage of protein to carbohydrate in the extracellular protein-polysaccharide from the red alga Porphyridium cruentum. Biochemistry 1977, 165, 1–9. [Google Scholar]
- Shrestha, R.; Bar-Zvi, D.; Arad, S.M. Non-Covalently Bound Cell-Wall Proteins of the Red Microalga Porphyridium sp. In Proceedings of7th Cell-Wall Meeting, Santiago, Spain, 25–29 September 1995; p. 114.
- Shrestha, R.P.; Weinstein, Y.; Bar-Zvi, D.; Arad, S.M. A glycoprotein noncovalently associated with cell-wall polysaccharide of the red microalga Porphyridium sp. (Rhodophyta). J. Phycol. 2004, 40, 568–580. [Google Scholar] [CrossRef]
- Heaney-Kieras, J.; Swift, H. Electron microscopy of the extracellular protein-polysaccharide from the red alga Porphyridium cruentum. In Glycoconjugate Research; Jeanloz, R.W., Ed.; Academic Press: San Diego, CA, USA, 1979; pp. 165–166. [Google Scholar]
- Arad, S.M.; Adda, M.; Cohen, E. The potential of production of sulphated polysaccharides from Porphyridium. Plant Soil 1985, 89, 117–127. [Google Scholar] [CrossRef]
- Badel, S.; Callet, F.; Laroche, C.; Gardarin, C.; Petit, E.; el Alaoui, H.; Bernardi, T.; Michaud, P. A new tool to detect high viscous exopolymers from microalgae. J. Ind. Microbiol. Biotechnol. 2011, 38, 319–326. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, T.; Hayashi, K.; Sankawa, U. Structural analysis of calcium spirulan (Ca-SP)-derived oligosaccharides using electrospray inonization mass spectrometry. J. Nat. Prod. 2000, 63, 136–138. [Google Scholar] [CrossRef]
- A Structural View of Rheology. Available online: http://www.vilastic.com/tech4.html (accessed on 3 April 2012).
- Geresh, S.; Dawadi, R.P.; Arad, S.M. Chemical modifications of biopolymers: Quaternization of the extracellular polysaccharide of the red microalga Porphyridium sp. Carbohydr. Polym. 2000, 63, 75–80. [Google Scholar]
- Ginzberg, A.; Korin, E.; Arad, S.M. Effect of drying on the biological activities of a red microalga polysaccharide. Biotechnol. Bioeng. 2008, 99, 411–420. [Google Scholar] [CrossRef]
- Proksch, E.; Holleran, W.M.; Menon, G.K.; Elias, P.M.; Feingold, K.R. Barrier function regulates epidermal lipid and DNA synthesis. Br. J. Dermatol. 1993, 128, 473–482. [Google Scholar]
- Kim, M.; Yim, J.H.; Kim, S.-Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.-S.; Lee, C.-K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulphated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef]
- Xing, R.E.; Yu, H.H.; Liu, S. Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorg. Med. Chem. 2005, 13, 1387–1392. [Google Scholar] [CrossRef]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef]
- Bae, S.Y.; Yim, J.H.; Lee, H.K.; Pyo, S. Activation of murine peritoneal macrophages by sulphated exopolysaccharide from marine microalga Gyrodinium impudicum (strain KG03): Involvement of the NF-kappa B and JNK pathway. Int. Immunopharmacol. 2006, 6, 473–484. [Google Scholar] [CrossRef]
- Namikoshi, M. Bioactive compounds produced by cyanobacteria. J. Int. Microbiol. Biotechnol. 1996, 17, 373–384. [Google Scholar] [CrossRef]
- Yim, J.H.; Son, E.; Pyo, S.; Lee, H.K. Novel sulfated polysaccharide derived from red-tide microalga Gyrodinium impudicum strain KG03 with immunostimulating activity in vivo. Mar. Biotechnol. 2005, 7, 331–338. [Google Scholar] [CrossRef]
- Morais, M.G.; Stillings, C.; Dersch, R.; Rudisile, M.; Pranke, P.; Costa, J.A.V.; Wendorff, J. Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour. Technol. 2010, 101, 2872–2876. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, Y.; Ewart, H.S. Chemical structures and bioactives of sulphated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Dvir, I.; Maislos, M.; Arad, S.M. Feeding rodents with red microalgae. In Dietary Fiber, Mechanisms of Action in Human Physiology and Metabolism; Cherbut, C., Barry, J.L., Lairon, D., Durand, M., Eds.; John Libbey Eurotext: Paris, France, 1995; pp. 86–91. [Google Scholar]
- Oakenfull, D. Physicochemical properties of dietary fiber: Overview. In Handbook of Dietary Fibers; Cho, S.S., Dreher, M.D., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 195–206. [Google Scholar]
- Glore, S.R.; van Treeck, D.; Knehans, A.W.; Guild, M. Soluble fiber in serum lipids: A literature review. J. Am. Diet. Assoc. 1994, 94, 425–436. [Google Scholar] [CrossRef]
- Marlett, J. Dietary fibre and cardiovascular disease. In Handbook of Dietary Fibers; Cho, S.S., Dreher, M.D., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 17–30. [Google Scholar]
- Ofek, L.; Beachery, E.H.; Sharon, N. Surface sugars recognition in bacterial adherence. Trends Biochem. Sci. 1978, 3, 159–160. [Google Scholar] [CrossRef]
- Ascencio, F.; Fransson, L.A.; Wadström, T. Affinity of the gastric pathogen Helicobacter pylori for the N-sulphated glycosaminoglycan heparin sulphate. J. Med. Microbiol. 1993, 38, 240–244. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar]
- Pereira, M.S.; Vilela-Silva, A.C.; Valente, A.P.; Mourão, P.A. A 2-sulfated, 3-linked alpha-L-galactan is an anticoagulant polysaccharide. Carbohydr. Res. 2002, 337, 2231–2238. [Google Scholar]
- Arad, S.M.; Weinstein, J. Novel lubricants from red microalgae: Interplay between genes and products. J. Biomed. (Isr.) 2003, 1, 32–37. [Google Scholar]
- Tseng, C. Algal biotechnology industries and research activities in China. J. Appl. Phycol. 2001, 13, 375–380. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Halliwell, B. Antioxidants in Nutrition, Health, and Disease; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Wetherbee, R.; Lind, J.L.; Burke, J.; Quatrano, R.S. The first kiss: Establishment and control of initial adhesion by raphid diatoms. J. Phycol. 1998, 34, 9–15. [Google Scholar]
- Paterson, D.M. Short-Term changes in the erodibility of intertidal cohesive sediments related to the migratory behaviour of epipelic diatoms. Limnol. Oceanogr. 1989, 34, 223–234. [Google Scholar] [CrossRef]
- Sutherland, T.F.; Grant, J.; Amos, C.L. The effect of carbohydrate production by the diatom Nitzschia curvilineata on the erodibility of sediment. Limnol. Oceanogr. 1998, 43, 65–72. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Raposo, M.F.d.J.; De Morais, R.M.S.C.; Bernardo de Morais, A.M.M. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233-252. https://doi.org/10.3390/md11010233
Raposo MFdJ, De Morais RMSC, Bernardo de Morais AMM. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Marine Drugs. 2013; 11(1):233-252. https://doi.org/10.3390/md11010233
Chicago/Turabian StyleRaposo, Maria Filomena de Jesus, Rui Manuel Santos Costa De Morais, and Alcina Maria Miranda Bernardo de Morais. 2013. "Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae" Marine Drugs 11, no. 1: 233-252. https://doi.org/10.3390/md11010233
APA StyleRaposo, M. F. d. J., De Morais, R. M. S. C., & Bernardo de Morais, A. M. M. (2013). Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Marine Drugs, 11(1), 233-252. https://doi.org/10.3390/md11010233