Dose-Response on the Chemopreventive Effects of Sarcophine-Diol on UVB-Induced Skin Tumor Development in SKH-1 Hairless Mice
Abstract
:1. Introduction
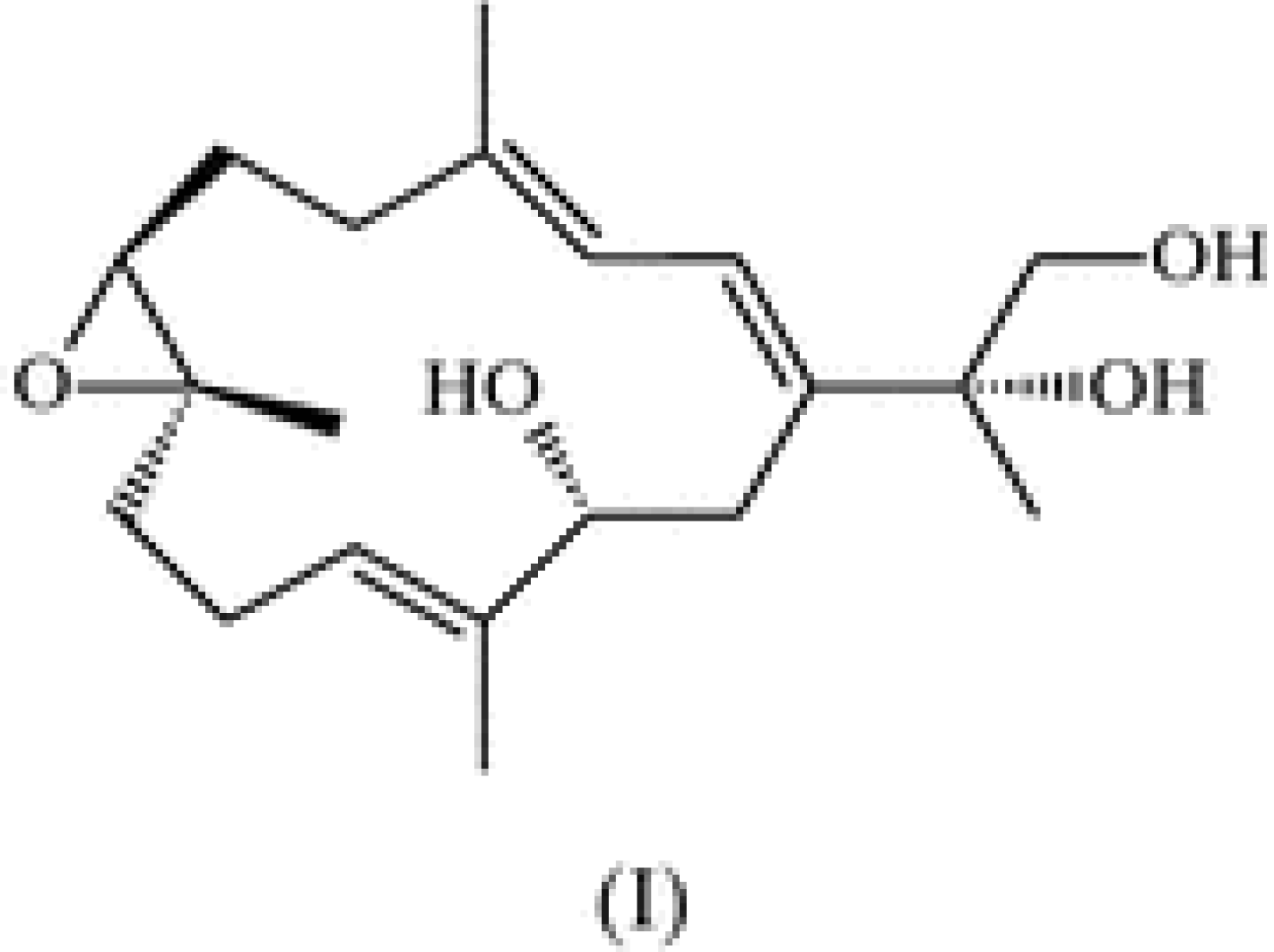
2. Results and Discussion
2.1. SD Treatment Did Not Affect Body Weight of SKH-1 Hairless Mice
2.2. SD Treatment Alone Did Not Affect the Skin of SKH-1 Hairless Mice
2.3. SD Treatment Did Not Significantly Inhibit the Incidence of Skin Tumors in SKH-1 Hairless Mice

2.4. SD Treatment Inhibited Tumor Multiplicity in SKH-1 Mice
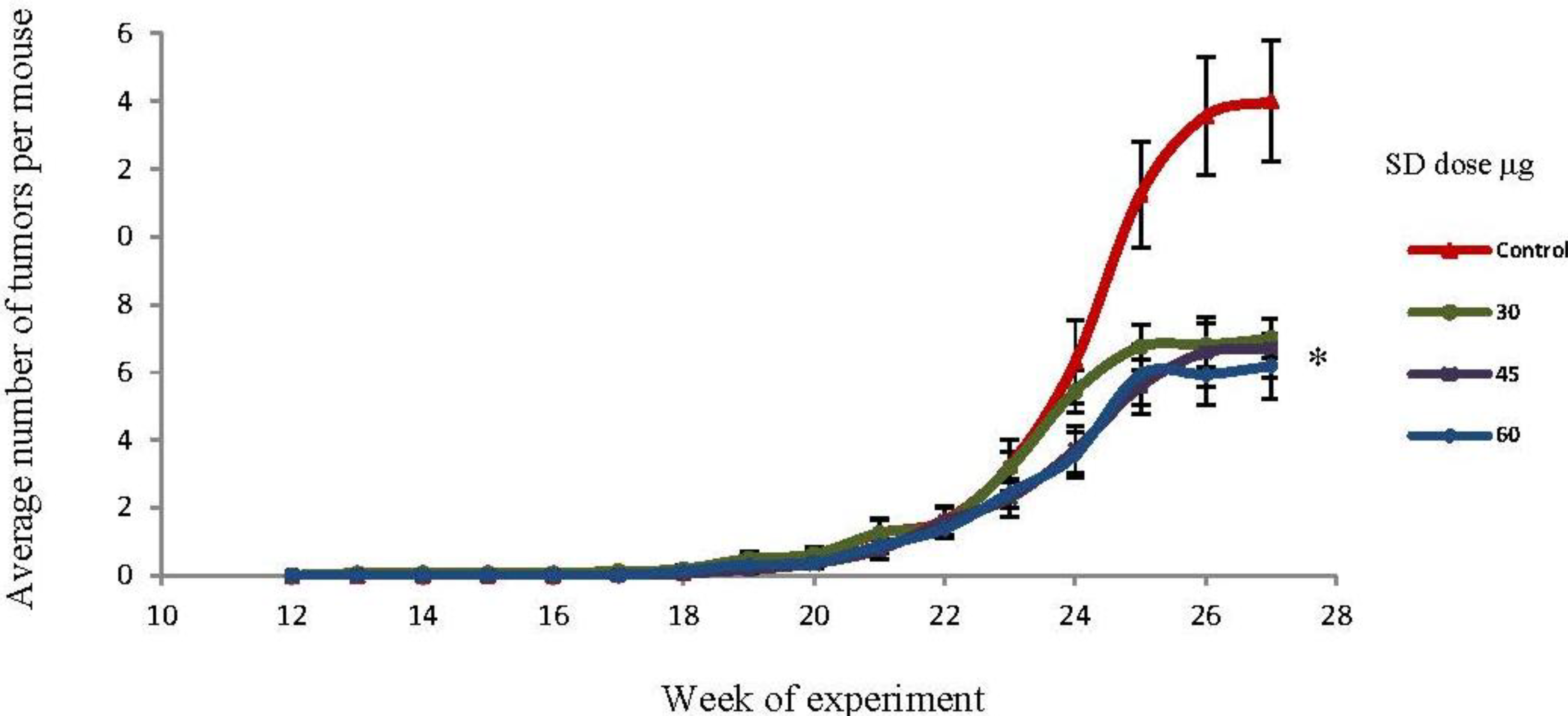
2.5. SD Treatment Inhibited Tumor Volume in SKH-1 Mice

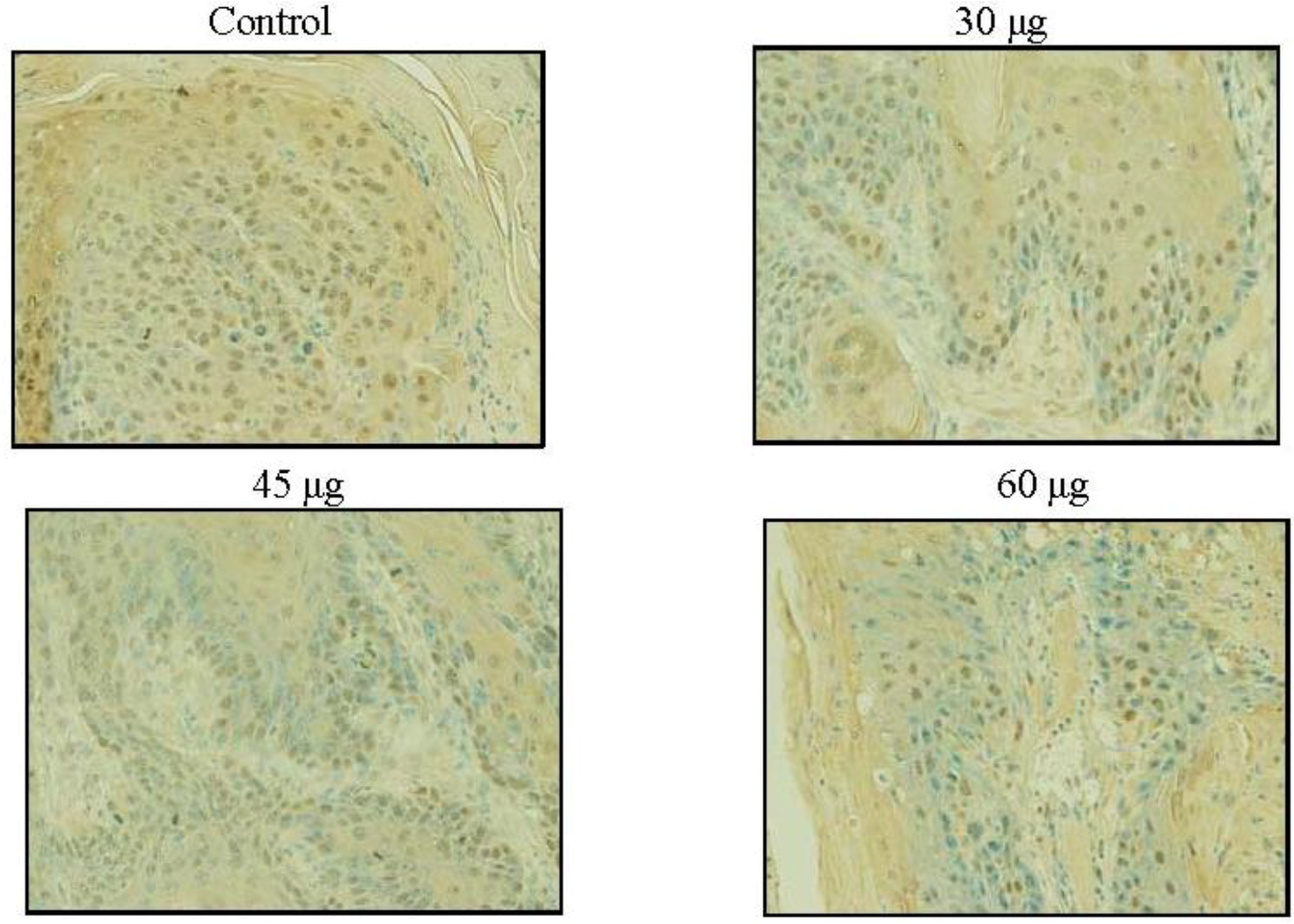
2.6. SD Inhibits UVB-Induced PCNA Positive Cells on Mice Skin

2.7. UVB Radiation Induced Carcinoma in Situ and Squamous Cell Carcinoma in SD Treated and Control Groups
2.8. Discussion
3. Experimental Section
3.1. Materials and Reagents
3.2. Synthesis of Sarcophine-Diol (SD)
3.3. Animals
3.4. UVBExposureSource
3.5. UVB-Induced Skin Carcinogenesis Protocol
3.6. Immunohistochemical Detection of PCNA-Positive Cells
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Skin Cancer Foundation. Available online: http://www.who.int/uv/faq/skincancer/en/index1.html (accessed on 20 July 2012).
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef]
- Kremer, K.H. Sunlight and skin cancer: Another link revealed. Proc. Natl. Acad. Sci. USA 1997, 94, 11–14. [Google Scholar] [CrossRef]
- Koh, H.K. Preventive strategies and research for ultraviolet-associated cancer. Environ. Health Perspect. 1995, 103, 255–257. [Google Scholar]
- Katiyar, S.K.; Korman, N.J.; Mukhtar, H.; Agarwal, R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997, 89, 556–566. [Google Scholar] [CrossRef]
- Learn, D.B.; Beasley, D.G.; Giddens, L.D.; Beard, J.; Stanfield, J.W.; Roberts, L.K. Minimum doses of ultraviolet radiation required to induce murine skin edema and immunosuppression are different and depend on the ultraviolet emission spectrum of the source. Photochem. Photobiol. 1995, 62, 1066–1075. [Google Scholar]
- De Gruijil, F.R. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol. Appl. Skin Physiol 2002, 15, 316–320. [Google Scholar] [CrossRef]
- Tyrell, R.M. Activation of mammalian gene expression by the UV component of sunlight-from models to reality. Bioessays 1996, 18, 139–148. [Google Scholar] [CrossRef]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–19. [Google Scholar] [CrossRef]
- Wolf, P.; Donawho, C.K.; Kripke, M.L. Effects of sunscreens on UV radiation-induced enhancement of melanoma growth in mice. J. Natl. Cancer Inst. 1994, 86, 99–105. [Google Scholar] [CrossRef]
- Schwartsmann, G.; Brondani da Rocha, A.; Berlinck, R.G.; Jimeno, J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001, 2, 221–225. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Russo, P.; Nastrucci, C.; Cesario, A. From the sea to anticancer therapy. Curr. Med. Chem. 2011, 18, 3551–3562. [Google Scholar] [CrossRef]
- Katsuyama, I.; Fahmy, H.; Zjawiony, J.K.; Khalifa, S.I.; Kilada, R.W.; Konoshima, T.; Takasaki, M.; Tokuda, H. Semisynthesis of new sarcophine derivatives with chemopreventive activity. J. Nat. Prod. 2002, 65, 1809–1814. [Google Scholar]
- Fahmy, H.; Khalifa, S.; Konoshima, T.; Zjawiony, J.K. An improved synthesis of 7,8-epoxy-1,3,11-cembratriene-15R(α),16-diol, a cembranoid of marine origin with a potent cancer chemopreventive activity. Mar. Drugs 2004, 2, 1–7. [Google Scholar]
- Fahmy, H.; Zjawiony, J.K.; Konoshima, T.; Tokuda, H.; Khan, S.; Khalifa, S. Potent skin cancer chemopreventing activity of some novel semi-synthetic cembranoids from marine sources. Mar. Drugs 2006, 4, 28–36. [Google Scholar] [CrossRef]
- Zhang, X.; Kundoor, V.; Khalifa, S.; Zeman, D.; Fahmy, H.; Dwivedi, C. Chemopreventive effects of sarcophine-diol on skin tumor development in CD-1 mice. Cancer Lett. 2007, 253, 53–59. [Google Scholar] [CrossRef]
- Zhang, X.; Bommareddy, A.; Chen, W.; Hildreth, M.B.; Kaushik, R.S.; Zeman, D.; Khalifa, S.; Fahmy, H.; Dwivedi, C. Chemopreventive effects of sarcophine-diol on ultraviolet B-induced skin tumor development in SKH-1 hairless mice. Mar. Drugs 2009, 7, 153–165. [Google Scholar] [CrossRef]
- Zhang, X.; Bommareddy, A.; Chen, W.; Khalifa, S.; Kaushik, R.S.; Fahmy, H.; Dwivedi, C. Sarcophine-diol, a chemopreventive agent of skin cancer, inhibits cell growth and induces apoptosis through extrinsic pathway in human epidermoid carcinoma A431 cells. Transl. Oncol. 2009, 2, 21–30. [Google Scholar]
- Szymanski, P.T.; Kuppast, B.; Ahmed, S.A.; Khalifa, S.; Fahmy, H. Sarcophine-diol, a skin cancer chemopreventive agent, inhibits proliferation and stimulates apoptosis in mouse melanoma B(1)(6)F(1)(0) cell line. Mar. Drugs 2012, 10, 1–19. [Google Scholar]
- Wood, R.D.; Shivji, M.K. Which DNA polymerases are used for DNA-repair in eukaryotes? Carcinogenesis 1997, 18, 605–610. [Google Scholar] [CrossRef]
- Shivji, K.K.; Kenny, M.K.; Wood, R.D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell 1992, 69, 367–374. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Qian, L.; Choi, C.I.; Steele, V.E.; Rao, C.V. Chemopreventive effects of RXR-selective rexinoid bexarotene on intestinal neoplasia of ApcMin/+ Mice. Neoplasia 2012, 14, 159–168. [Google Scholar]
- Smolarek, A.K.; So, J.Y.; Thomas, P.E.; Lee, H.J.; Paul, S.; Dombrowski, A.; Wang, C.X.; Saw, C.L.; Khor, T.O.; Kong, A.N.; et al. Dietary tocopherols inhibit cell proliferation, regulate expression of ERalpha, PPARgamma, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Mol. Carcinog. 2012. [Google Scholar]
- Saida, T.; Dohi, S.; Sadaki, M.; Tokuda, Y.; Ikegawa, S.; Takasaki, Y. Distribution patterns and frequency of proliferating cells in cutaneous keratinocytic neoplasms. Immunohistochemical study with a monoclonal antibody (TOB7) used against proliferating cell nuclear antigen. J. Am. Acad. Dermatol. 1992, 26 (5 Pt. 1), 744–748. [Google Scholar]
- Kawahira, K. Immunohistochemical staining of proliferating cell nuclear antigen (PCNA) in malignant and nonmalignant skin diseases. Arch. Dermatol. Res. 1999, 291, 413–418. [Google Scholar] [CrossRef]
- Toth, D.P.; Guenther, L.C.; Shum, D.T. Proliferating cell nuclear antigen (PCNA); prognostic value in the clinical recurrence of primary basal cell carcinoma. J. Dermatol. Sci. 1996, 11, 36–40. [Google Scholar] [CrossRef]
- Lu, Y.P.; Lou, Y.R.; Xie, J.G.; Peng, Q.Y.; Liao, J.; Yang, C.S.; Huang, M.T.; Conney, A.H. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 12455–12460. [Google Scholar]
- Chilampalli, C.; Guillermo, R.; Zhang, X.; Kaushik, R.S.; Young, A.; Zeman, D.; Hildreth, M.B.; Fahmy, H.; Dwivedi, C. Effects of magnolol on UVB-induced skin cancer development in mice and its possible mechanism of action. BMC Cancer 2011, 11, 456. [Google Scholar] [CrossRef]
- Chilampalli, S.; Zhang, X.; Fahmy, H.; Kaushik, R.S.; Zeman, D.; Hildreth, M.B.; Dwivedi, C. Chemopreventive effects of honokiol on UVB-induced skin cancer development. Anticancer Res. 2010, 30, 777–783. [Google Scholar]
- Moore, J.O.; Palep, S.R.; Saladi, R.N.; Gao, D.; Wang, Y.; Phelps, R.G.; Lebwohl, M.G.; Wei, H. Effects of ultraviolet B exposure on the expression of proliferating cell nuclear antigen in murine skin. Photochem. Photobiol. 2004, 80, 587–595. [Google Scholar]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef]
- Bickers, D.R.; Lim, H.W.; Margolis, D.; Weinstock, M.A.; Goodman, C.; Faulkner, E.; Gould, C.; Gemmen, E.; Dall, T. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J. Am. Acad. Dermatol. 2006, 55, 490–500. [Google Scholar] [CrossRef]
- Centers for Diseas Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. Morb. Mortal Wkly. Rep. 2012, 61, 323–326.
- Suganuma, M.; Okabe, S.; Sueoka, E.; Iida, N.; Komori, A.; Kim, S.J.; Fujiki, H. A new process of cancer prevention mediated through inhibition of tumor necrosis factor alpha expression. Cancer Res. 1996, 56, 3711–3715. [Google Scholar]
- Fujiki, H.; Suganuma, M.; Suguri, H.; Yoshizawa, S.; Takagi, K.; Kobayashi, M. Sarcophytols A and B inhibit tumor promotion by teleocidin in two-stage carcinogenesis in mouse skin. J. Cancer Res. Clin. Oncol. 1989, 115, 25–28. [Google Scholar] [CrossRef]
- Yamauchi, O.; Omori, M.; Ninomiya, M.; Okuno, M.; Moriwaki, H.; Suganuma, M.; Fujiki, H.; Muto, Y. Inhibitory effect of sarcophytol A on development of spontaneous hepatomas in mice. Jpn. J. Cancer Res. 1991, 82, 1234–1238. [Google Scholar] [CrossRef]
- Steele, V.E.; Moon, R.C.; Lubet, R.A.; Grubbs, C.J.; Reddy, B.S.; Wargovich, M.; McCormick, D.L.; Pereira, M.A.; Crowell, J.A.; Bagheri, D.; et al. Preclinical efficacy evaluation of potential chemopreventive agents in animal carcinogenesis models: Methods and results from the NCI Chemoprevention Drug Development Program. J. Cell. Biochem. Suppl. 1994, 20, 32–54. [Google Scholar]
- Ne’eman, I.; Fishelson, L.; Kashman, Y. Sarcophine—A new toxin from the soft coral Sarcophyton glaucum (Alcyonaria). Toxicon 1974, 12, 593–598. [Google Scholar] [CrossRef]
- Erman, A.; Neeman, I. Inhibition of phosphofructokinase by the toxic cembranolide sarcophine isolated from the soft-bodied coral Sarcophyton glaucum. Toxicon 1977, 15, 207–215. [Google Scholar] [CrossRef]
- Zhou, B.R.; Liu, W.L.; Luo, D. Protective effect of baicalin against multiple ultraviolet B exposure-mediated injuries in C57BL/6 mouse skin. Arch. Pharm. Res. 2011, 34, 261–268. [Google Scholar] [CrossRef]
- Ahsan, H.; Reagan-Shaw, S.; Eggert, D.M.; Tan, T.C.; Afaq, F.; Mukhtar, H.; Ahmad, N. Protective effect of sanguinarine on ultraviolet B-mediated damages in SKH-1 hairless mouse skin: Implications for prevention of skin cancer. Photochem. Photobiol. 2007, 83, 986–993. [Google Scholar] [CrossRef]
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.C.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749. [Google Scholar] [CrossRef]
- Zhang, X.; Dwivedi, C. Skin cancer chemoprevention by alpha-santalol. Front. Biosci. 2011, 3, 777–787. [Google Scholar] [CrossRef]
- Meeran, S.M.; Mantena, S.K.; Elmets, C.A.; Katiyar, S.K. (−)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006, 66, 5512–5520. [Google Scholar] [CrossRef]
- Gu, M.; Singh, R.P.; Dhanalakshmi, S.; Agarwal, C.; Agarwal, R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007, 67, 3483–3491. [Google Scholar]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guillermo, R.F.; Zhang, X.; Kaushik, R.S.; Zeman, D.; Ahmed, S.A.; Khalifa, S.; Fahmy, H.; Dwivedi, C. Dose-Response on the Chemopreventive Effects of Sarcophine-Diol on UVB-Induced Skin Tumor Development in SKH-1 Hairless Mice. Mar. Drugs 2012, 10, 2111-2125. https://doi.org/10.3390/md10092111
Guillermo RF, Zhang X, Kaushik RS, Zeman D, Ahmed SA, Khalifa S, Fahmy H, Dwivedi C. Dose-Response on the Chemopreventive Effects of Sarcophine-Diol on UVB-Induced Skin Tumor Development in SKH-1 Hairless Mice. Marine Drugs. 2012; 10(9):2111-2125. https://doi.org/10.3390/md10092111
Chicago/Turabian StyleGuillermo, Ruth F., Xiaoying Zhang, Radhey S. Kaushik, David Zeman, Safwat A. Ahmed, Sherief Khalifa, Hesham Fahmy, and Chandradhar Dwivedi. 2012. "Dose-Response on the Chemopreventive Effects of Sarcophine-Diol on UVB-Induced Skin Tumor Development in SKH-1 Hairless Mice" Marine Drugs 10, no. 9: 2111-2125. https://doi.org/10.3390/md10092111



