Pigmentation and Spectral Absorbance Signatures in Deep-Water Corals from the Trondheimsfjord, Norway
Abstract
:1. Introduction
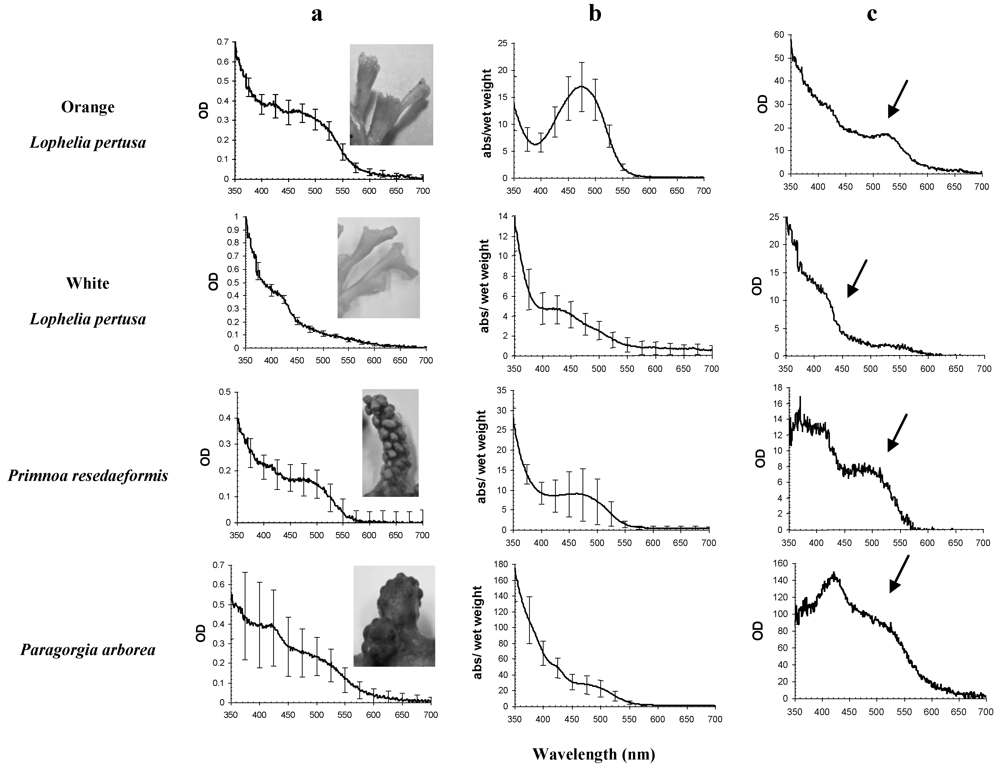
2. Results and Discussion
| In vivo | In vitro | ||||
|---|---|---|---|---|---|
| Species | N | Mean λmax | CV% | Mean | CV% |
| Orange Lophelia pertusa | 3 | 473 | 0.88 | 477 | 0.12 |
| White Lophelia pertusa | 3 | 409 | 0.61 | 429 | 0.36 |
| Paragorgia arborea | 3 | 475 | 1.16 | 475 | 0.76 |
| Primnoa resedaeformis | 3 | 476 | 0.32 | 463 | 2.27 |


| Pigment | Retention time (min) | Orange L. pertusa | White L. pertusa | P. arborea | P. resedaeformis |
|---|---|---|---|---|---|
| Astaxanthin | 21 | X | X | X | X |
| Canthaxanthin-like | 25–26 | Traces | Traces | X | Traces |
| Unknown pigments | 5, 12, 41 | - | - | X | - |
| Pigments in solvent front | 1–2 | X | X | X | X |
| Species | Astaxanthin | |||
|---|---|---|---|---|
| λmax (nm) in methanol | ng astaxanthin ng−1 coral sample | Accurate mass (M + H)+ | ppm error | |
| Orange Lophelia pertusa | 476 | 3.75 × 10−7 | 597.3940 | 0.32 |
| White Lophelia pertusa | 475 | 1.65 × 10−7 | 597.3936 | −0.35 |
| Paragorgia arborea | 478 | 3.15 × 10−6 | 597.3938 | 0 |
| Primnoa resedaeformis | 473 | 1.37 × 10−7 | Pigments degraded | - |
3. Experimental Section
3.1. Study Area and Sample Collection
3.2. Absorption Characteristics and Pigment Extraction
3.3. Pigment Analysis
4. Conclusions
Acknowledgments
References
- Roberts, J.M.; Wheeler, A.J.; Freiwald, A. Reefs of the deep: The biology and geology of cold-water coral ecosystems. Science 2006, 312, 543–547. [Google Scholar] [CrossRef]
- Wheeler, A.J.; Beyer, A.; Freiwald, A.; de Haas, H.; Huvenne, V.A.I.; Kozachenko, M.; Olu-Le Roy, K.; Opderbecke, J. Morphology and environment of cold-water coral carbonate mounds on the NW European margin. Int. J. Earth Sci. 2007, 96, 37–56. [Google Scholar] [CrossRef]
- Fosså, J.H.; Lindberg, B.; Christensen, O.; Lundälv, T.; Svellingen, I.; Mortensen, P.B.; Alvsvåg, J. Mapping of Lophelia Reefs in Norway: Experiences and Survey Methods. In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Springer: Berlin, Heidelberg, Germany, 2005; pp. 359–391. [Google Scholar]
- Mortensen, P.B.; Buhl-Mortensen, L. Distribution of deep-water gorgonian corals in relation to benthic habitat features in the Northeast Channel (Atlantic Canada). Mar. Biol. 2004, 144, 1223–1238. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Buhl-Mortensen, L. Morphology and growth of the deep-water gorgonians Primnoa resedaeformis and Paragorgia arborea. Mar. Biol. 2005, 147, 775–788. [Google Scholar] [CrossRef]
- Freiwald, A.; Roberts, J.M.; Mortensen, P.B.; Buhl-Mortensen, L. Deep-Water Corals and their Habitats in The Gully, a Submarine Canyon off Atlantic Canada. In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Springer: Berlin, Heidelberg, Germany, 2005; pp. 247–277. [Google Scholar]
- Madsen, F.J. Octocorallia:Stolinifera, Telestacea, Xeniidea, Alcyonacea, Gorgonacea. In The Danish Ingolf-Expedition; H. Hagerup: Copenhagen, Denmark, 1944; Volume 13. [Google Scholar]
- Tendal, O.S. The North Atlantic distribution of the octocoral Paragorgia arborea (L., 1758) (Cnidaria, Anthozoa). Sarsia 1992, 77, 213–217. [Google Scholar]
- Cairns, S.D.; Bayer, F.M. A review of the genus Primnoa (Octocorallia: Gorgonacea: Primnoidae), with the description of two new species. Bull. Mar. Sci. 2005, 77, 225–256. [Google Scholar]
- Hovland, M.; Mortensen, P.B. Norske Korallrev og Prosesser i Havbunnen; John Grieg forlag: Bergen, Norway, 1999. [Google Scholar]
- Anthony, K.R.N.; Fabricius, K.E. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 2000, 252, 221–253. [Google Scholar] [CrossRef]
- Costello, M.J.; McCrea, M.; Freiwald, A.; Lundälv, T.; Jonsson, L.; Bett, B.J.; van Weering, T.; de Haas, H.; Roberts, J.M.; Allen, D. Role of Cold-Water Lophelia pertusa Coral Reefs as Fish Habitat in the NE Atlantic. In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Springer: Berlin, Heidelberg, Germany, 2005; pp. 771–805. [Google Scholar]
- van Weering, T.C.E.; Duineveld, G.; van Duyl, F.; Lavaleye, M.; Mienis, F.; Maier, C.; de Haas, H.; van der Land, C. Cold Water Corals and Carbonate Mounds. In Annual Report. Royal Netherlands Institute for Sea Research; Royal Netherlands Institute for Sea Research: Texel, The Netherlands, 2006; pp. 9–12. [Google Scholar]
- Sherwood, O.A.; Jamieson, R.E.; Edinger, E.N.; Wareham, V.E. Stable C and N isotopic composition of cold-water corals from the Newfoundland and Labrador continental slope: Examination of trophic, depth and spatial effects. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 1392–1402. [Google Scholar] [CrossRef]
- Freiwald, A.; Hühnerbach, V.; Lindberg, B.; Wilson, J.B.; Campbell, J. The Sula reef complex, Norwegian shelf. Facies 2002, 47, 179–200. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. The nature and role of pigments of marine invertebrates. Nat. Prod. Rep. 2006, 23, 223–255. [Google Scholar] [CrossRef]
- Waller, R.G.; Tyler, P.A. The reproductive biology of two deep-water, reef-building scleractinians from the NE Atlantic Ocean. Coral Reefs 2005, 24, 514–522. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar]
- Matsuno, T. Aquatic animal carotenoids. Fisheries Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Goodwin, T.W. Chemistry and Biochemistry of Plant Pigments, 2nd ed; Academic Press: Cambridge, MA, USA, 1976; Volume 1. [Google Scholar]
- Straub, O. Key to Carotenoids, 2nd ed; Birkhäuser Verlag: Basel, Switzerland, 1987. [Google Scholar]
- Johnsen, G.; Sakshaug, E. Biooptical characteristics of PSII and PSI in 33 species (13 pigment groups) of marine phytoplankton, and the relevance for pulse-amplitude-modulated and fast-repetition-rate fluorometry. J. Phycol. 2007, 43, 1236–1251. [Google Scholar] [CrossRef]
- Roy, S.; Egeland, E.S.; Llewellyn, C.; Johnsen, G. Phytoplankton Pigments: Updates on Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011; p. 600. [Google Scholar]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar]
- Shnit-Orland, M.; Kushmaro, A. Coral Mucus Bacteria as a Source for Antibacterial Activity. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008.
- Paanakker, J.E.; Hallegraeff, G.M. A comparative study on carotenoid pigmentation of the zooplankton of lake Maarsseveen (Netherlands) and of lac Pavin (Auvergne, France)—I. Chromatographic characterization of carotenoid pigments. Comp. Biochem. Physiol. B 1978, 60, 51–58. [Google Scholar] [CrossRef]
- Cvejic, J.; Tambutté, S.; Lotto, S.; Mikov, M.; Slacanin, I.; Allemand, D. Determination of canthaxanthin in the red coral (Corallium rubrum) from Marseille by HPLC combined with UV and MS detection. Mar. Biol. 2007, 152, 855–862. [Google Scholar] [CrossRef]
- Tanaka, Y.; Matsuguchi, H.; Katayama, T.; Simpson, K.L.; Chichester, C.O. Biosynthesis of astaxanthin—XVI. Carotenoids in crustacea. Comp. Biochem. Physiol. B 1976, 54, 391–393. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Gentien, P. Carotenoids of Temora turbinata, Centropages furcatus, Undinula vulgaris and Euchaeta russelli. Comp. Biochem. Physiol. B 1982, 72, 409–414. [Google Scholar] [CrossRef]
- Upadhyay, R.R.; Liaaen-Jensen, S. Animal carotenoids. 5. Carotenoids of some Anthozoa. Acta Chem. Scand. 1970, 24, 3055–3057. [Google Scholar] [CrossRef]
- Thoen, H.; Johnsen, G.; Berge, J. Pigmentation and spectral absorbance in the deep-sea arctic amphipods Eurythenes gryllus and Anonyx sp. Polar Biol. 2010, 34, 83–93. [Google Scholar]
- Johnsen, G.; Nelson, N.B.; Jovine, R.V.M.; Prezelin, B.B. Chromoprotein- and pigment-dependent modelling of spectral light absorption in two dinoflagellates, Prorocentrum minimum and Heterocapsa pygmaea. Mar. Ecol. Prog. Ser. 1994, 114, 245–258. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; MacTavish, H.S.; Dunlap, W.C.; Vesk, M.; Groenewoud, K. Occurrence of UVA- and UVB-absorbing compounds in 152 species (206 strains) of marine microalgae. Mar. Ecol. Prog. Ser. 1999, 189, 35–51. [Google Scholar] [CrossRef]
- Rønneberg, H.; Fox, D.L.; Liaaen-Jensen, S. Animal carotenoids-carotenoproteins from hydrocorals. Comp. Biochem. Physiol. B 1979, 64, 407–408. [Google Scholar] [CrossRef]
- Czeczuga, B. Investigations of carotenoprotein complexes in animals—VI. Anemonia sulcata, the representative of askeletal coral. Comp. Biochem. Physiol. B 1983, 75, 181–183. [Google Scholar] [CrossRef]
- Fox, D.L.; Wilkie, D.W. Somatic and skeletally fixed carotenoids of the purple hydrocoral, Allopora californica. Comp. Biochem. Physiol. 1970, 36, 49–60. [Google Scholar] [CrossRef]
- Tolasa, S.; Cakli, S.; Ostermeyer, U. Determination of astaxanthin and canthaxanthin in salmonid. Eur. Food Res. Technol. 2005, 221, 787–791. [Google Scholar] [CrossRef]
- Roberts, J.M.; Wheeler, A.; Freiwald, A.; Cairns, S. Cold-Water Corals: The Biology and Geology of Deep-Sea Coral Habitats; Cambridge University Press: Cambridge, UK, 2009; p. 334. [Google Scholar]
- van Nieuwerburgh, L.; Wänstrand, I.; Liu, J.; Snoeijs, P.L. Astaxanthin production in marine pelagic copepods grazing on two different phytoplankton diets. J. Sea Res. 2005, 53, 147–160. [Google Scholar] [CrossRef]
- Juan, F.M.; Gudiña, E.; Barredo, J. Conversion of β-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein? Microb. Cell Fact. 2008, 7, 1–10. [Google Scholar] [CrossRef]
- Neulinger, S.C.; Järnegren, J.; Ludvigsen, M.; Lochte, K.; Dullo, W.C. Phenotype-specific bacterial communities in the cold-water coral Lophelia pertusa (Scleractinia) and their implications for the coral’s nutrition, health, and distribution. Appl. Environ. Microbiol. 2008, 74, 7272–7285. [Google Scholar] [CrossRef] [Green Version]
- Neulinger, S.C.; Gärtner, A.; Järnegren, J.; Ludvigsen, M.; Lochte, K.; Dullo, W.C. Tissue-associated “Candidatus Mycoplasma corallicola” and filamentous bacteria on the cold-water coral Lophelia pertusa (Scleractinia). Appl. Environ. Microbiol. 2009, 75, 1437–1444. [Google Scholar]
- Kellogg, C.A.; Järnegren, J. Personal communication, 2012. Norwegian Institute for Nature Research, Trondheim, Norway..
- LeBoeuf, R.D.; McCommas, S.A.; Howe, N.R.; Tauber, J.D. The role of carotenoids in the color polymorphism of the sea anemone, Bunodosoma granulifera (Anthozoa, Actiniaria). Comp. Biochem. Physiol. 1981, 68B, 25–29. [Google Scholar]
- Fox, D.L.; Pantin, C.F.A. The Colours of the Plumose Anemone Metridium senile (L.). Phil. Trans. R. Soc. Lond. B 1941, 230, 415–450. [Google Scholar] [CrossRef]
- Yentsch, C.S. Measurement of visible light absorption by particulate matter in the ocean. Limnol. Oceanogr. 1962, 7, 207–217. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Kiefer, D.A. Chlorophyll a specific absorption and fluorescence excitation spectra for light-limited phytoplankton. Deep Sea Res. 1988, 35, 639–663. [Google Scholar] [CrossRef]
- Rodríguez, F.; Chauton, M.; Johnsen, G.; Andresen, K.; Olsen, L.M.; Zapata, M. Photoacclimation in phytoplankton: Implications for biomass estimates, pigment functionality and chemotaxonomy. Mar. Biol. 2006, 148, 963–971. [Google Scholar] [CrossRef]
- Stafsnes, M.H.; Josefsen, K.D.; Kildahl-Andersen, G.; Valla, S.; Ellingsen, T.E.; Bruheim, P. Isolation and characterization of marine pigmented bacteria from Norwegian coastal waters and screening for carotenoids with UVA-Blue light absorbing properties. J. Microbiol. 2010, 48, 16–23. [Google Scholar] [CrossRef]
- Bredholt, H.; Fjærvik, E.; Johnsen, G.; Zotchev, S.B. Actinomycetes from Sediments in the Trondheim Fjord, Norway: Diversity and biological activity. Mar. Drugs 2008, 6, 12–24. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycooela, F.M. Astaxanthin: a review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Cornet, S.; Biard, C.; Moret, Y. Is there a role for antioxidant carotenoids in limiting self-harming immune response in invertebrates? Biol. Lett. 2007, 3, 284–288. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Elde, A.C.; Pettersen, R.; Bruheim, P.; Järnegren, J.; Johnsen, G. Pigmentation and Spectral Absorbance Signatures in Deep-Water Corals from the Trondheimsfjord, Norway. Mar. Drugs 2012, 10, 1400-1411. https://doi.org/10.3390/md10061400
Elde AC, Pettersen R, Bruheim P, Järnegren J, Johnsen G. Pigmentation and Spectral Absorbance Signatures in Deep-Water Corals from the Trondheimsfjord, Norway. Marine Drugs. 2012; 10(6):1400-1411. https://doi.org/10.3390/md10061400
Chicago/Turabian StyleElde, Anette C., Ragnhild Pettersen, Per Bruheim, Johanna Järnegren, and Geir Johnsen. 2012. "Pigmentation and Spectral Absorbance Signatures in Deep-Water Corals from the Trondheimsfjord, Norway" Marine Drugs 10, no. 6: 1400-1411. https://doi.org/10.3390/md10061400




