Kiamycin, a Unique Cytotoxic Angucyclinone Derivative from a Marine Streptomyces sp.
Abstract
:1. Introduction
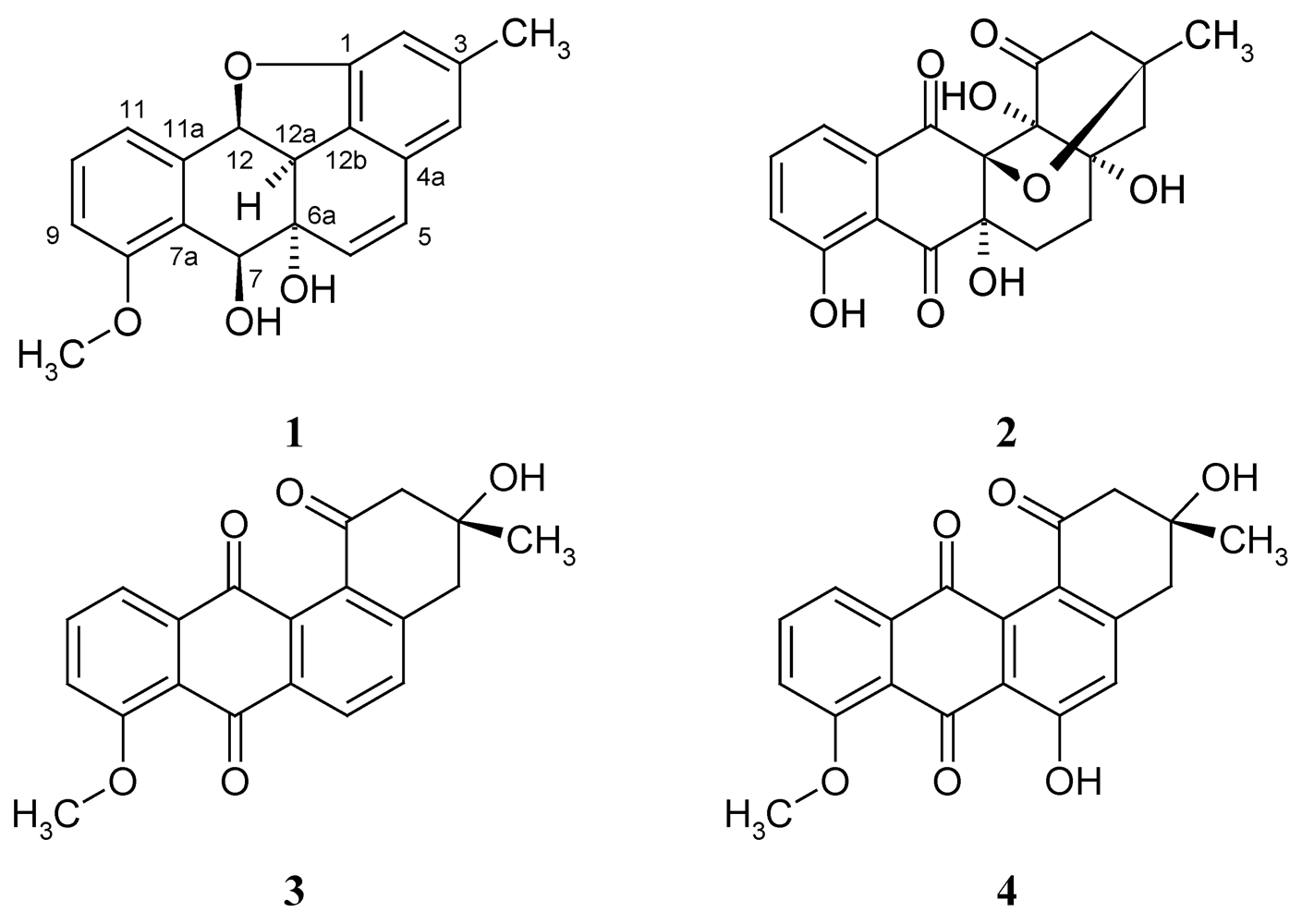
2. Results and Discussion
2.1. Isolation and Structure Elucidation of Kiamycin
 = –113.9; c 0.01, MeOH); the molecular formula was established as C20H18O4 by HRESIMS of the pseudomolecular ion peak at m/z 345.1103 [M + Na]+ (calcd for C20H18O4Na: 345.1097). The 1H NMR and COSY spectra indicated a 1,2,3-trisubstituted benzene ring by signals of o-coupled protons at δ 6.85 (d), 7.18 (d), and 7.38 (t); the expected m-coupling was not visible but was indicated by a line broadening and was visible in the COSY spectrum. A second benzene unit was connected with a methyl group (δ 2.24, s), which was flanked by two protons (broadened singlets at δ 6.41 and 6.50), as indicated by long-range couplings among each other and with the methyl group, respectively, and by NOE correlations with 3-Me. The 9.5 Hz coupling constant of two further doublets at δ 5.88 and 6.74 indicated a cis-configured double bond. Finally, a methoxy singlet appeared at δ 3.80, and signals of three oxymethine protons were seen at δ 4.11 (d), 4.85 (d), and 6.00 (d); the residual two hydrogen atoms should belong to hydroxy groups.
= –113.9; c 0.01, MeOH); the molecular formula was established as C20H18O4 by HRESIMS of the pseudomolecular ion peak at m/z 345.1103 [M + Na]+ (calcd for C20H18O4Na: 345.1097). The 1H NMR and COSY spectra indicated a 1,2,3-trisubstituted benzene ring by signals of o-coupled protons at δ 6.85 (d), 7.18 (d), and 7.38 (t); the expected m-coupling was not visible but was indicated by a line broadening and was visible in the COSY spectrum. A second benzene unit was connected with a methyl group (δ 2.24, s), which was flanked by two protons (broadened singlets at δ 6.41 and 6.50), as indicated by long-range couplings among each other and with the methyl group, respectively, and by NOE correlations with 3-Me. The 9.5 Hz coupling constant of two further doublets at δ 5.88 and 6.74 indicated a cis-configured double bond. Finally, a methoxy singlet appeared at δ 3.80, and signals of three oxymethine protons were seen at δ 4.11 (d), 4.85 (d), and 6.00 (d); the residual two hydrogen atoms should belong to hydroxy groups. | Position | δC, assignm. | δH, mult. ( J in Hz) | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 156.7, Cq | |||
| 2 | 110.5, CH | 6.41, s br | H-4, 3-CH3 | C-1, C-4, C-12b, 3-CH3 |
| 3 | 140.1, Cq | |||
| 4 | 117.6, CH | 6.50, s br | H-2, 3-CH3 | C-2, C-5, C-12b, 3-CH3 |
| 4a | 131.5, Cq | |||
| 5 | 130.0, CH | 6.74, d (9.5) | H-6 | C-4, C-4a, C-6a, C-12b |
| 6 | 134.9, CH | 5.88, d (9.5) | H-5 | C-4a, C-12a |
| 6a | 75.8, Cq | |||
| 7 | 66.4, CH | 4.85, d (1.5) | H-12a | C-6a, C-7a, C-8, C-11a, C-12a |
| 7a | 125.5, Cq | |||
| 8 | 158.3, Cq | |||
| 9 | 111.0, CH | 6.85, d (8.3) | H-10, H-11 | C-7a, C-8, C-11 |
| 10 | 130.1, CH | 7.38, t (7.9) | H-9, H-11 | C-8, C-11a |
| 11 | 123.3, CH | 7.18, d (7.9) | H-9, H-10 | C-7a, C-9, C-12 |
| 11a | 133.0, Cq | |||
| 12 | 82.6, CH | 6.00, d (10.4) | H-12a | C-1, C-6a, C-7a, C-11, C-11a, C-12a, C-12b |
| 12a | 44.0, CH | 4.11, d (10.4) | H-7, H-12 | C-6a, C-7, C-11a, C-12b |
| 12b | 122.0, Cq | |||
| 8-OCH3 | 55.9, CH3 | 3.80, s | C-8 | |
| 3-CH3 | 22.0, CH3 | 2.24, s | H-2, H-4 | C-2, C-3, C-4 |
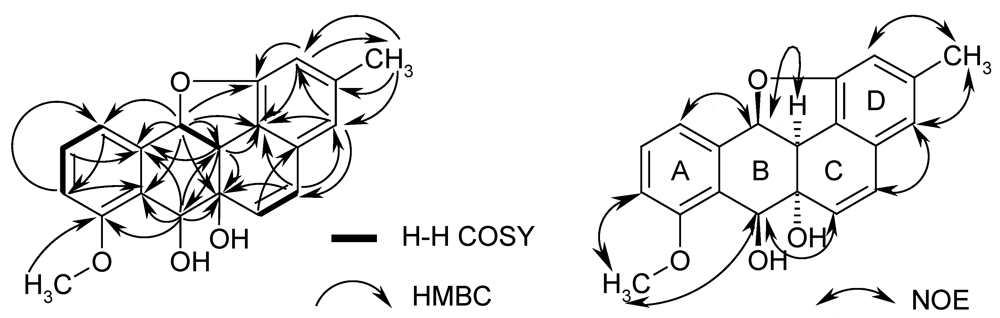
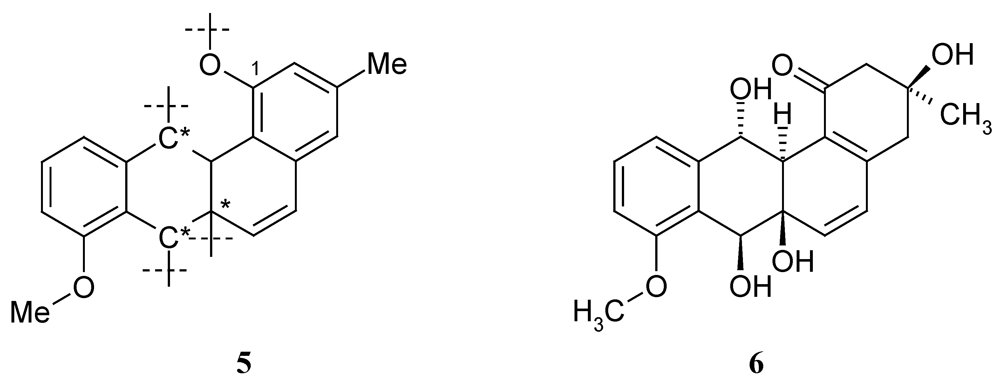

2.2. Biological Activity
| Cancer Cell Line | Inhibition Rate by 1 (%) | Inhibition Rate by Adriamycin (%) |
|---|---|---|
| human leukemic cell line HL-60 | 68.8 | 70.0 |
| human hepatoma cell line BEL-7402 | 31.7 | 79.3 |
| human lung adenocarcinoma cell line A549 | 55.9 | 80.8 |
3. Experimental Section
3.1. General
3.2. Isolation of the Producing Strain
3.3. Fermentation and Isolation
3.5. Cytotxicity Tests
4. Conclusions
Acknowledgments
References and Notes
- Fotso, S.; Fotso-Fondja Yao, C.B.; Helmke, E.; Laatsch, H. 2-Hydroxy-luisol A, a new quinone-derived tetraol from a marine Streptomyces sp. and oxidation products of luisol A. Z. Naturforsch. 2011, 66b, 629–634. [Google Scholar]
- Rohr, J.; Thiericke, R. Angucycline group antibiotics. Nat. Prod. Rep. 1992, 9, 103–137. [Google Scholar]
- Krohn, K.; Rohr, J. Angucyclines: Total syntheses, new structures, and biosynthetic studies of an emerging new class of antibiotics. Top. Curr. Chem. 1997, 188, 127–195. [Google Scholar] [CrossRef]
- Kharel, M.K.; Pahari, P.; Shepherd, M.D.; Tibrewal, N.; Nybo, S.E.; Shaaban, K.A.; Rohr, J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012, 29, 264–325. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Maksimenka, K.; Hamm, A.; Gulder, T.A.M.; Dieter, A.; Bull, A.T.; Stach, J.E.M.; Kocher, N.; Mueller, W.E.G.; et al. Gephyromycin, the first bridged angucyclinone, from Streptomyces griseus strain NTK 14. Phytochemistry 2005, 66, 1366–1373. [Google Scholar]
- Shigihara, Y.; Koizumi, Y.; Tamamura, T.; Homma, Y.; Isshiki, K.; Dobashi, K.; Naganawa, H.; Takeuchi, T. 6-Deoxy-8-O-methylrabelomycin and 8-O-methylrabelomycin from a Streptomyces species. J. Antibiot. 1988, 41, 1260–1264. [Google Scholar] [CrossRef]
- Asolkar, R.; Laatsch, H. Unpublished results. Data of X-14881F (6): C20H22O6; 1H NMR (DMSO-d6): 7.29 (t, J = 8 Hz, 1H, 10-H), 7.01 (d, J = 8.3 Hz; 1H, 9-H), 6.92 (d, J = 7.3 Hz; 1H, 11-H), 6.33 (d, J = 9.5 Hz; 1H, 6-H), 6.05 (d, J = 9.5 Hz; 1H, 5-H), 5.48 (d, J = 6.6 Hz, 1H, 7-OH), 5.24 (d, J = 9.5 Hz, 1H, 12-OH), 5.14 (s, 1H, 6a-OH), 4.88 (d, J = 9.5 Hz, 1H, 12-H), 4.85 (m, 1H, 7-H), 3.82 (s, 3H, 8-OMe), 2.69 (s br, 1H, 12a-H), 2.64, 2.54 (2 m, 2H, CH2-4), 2.58, 2.35 (AB, J = 15.9 Hz; 2H, CH2-2), 1.22 (s, 3H, 3-Me); 13C NMR (DMSO-d6): 197.5 (Cq-1), 52.4 (CH2-2), 69.7 (Cq-3), 44.3 (CH2-4), 146.4 (Cq-4a), 124.7 (CH-5), 138.7 (CH-6), 71.4 (Cq-6a), 67.7 (CH-7), 127.4 (Cq-7a), 156.8 (Cq-8), 111.0 (CH-9), 128.5 (CH-10), 119.6 (CH-11), 142.5 (Cq-11a), 70.1 (CH-12), 46.6 (Cq-12a), 125.8 (Cq-12b), 28.4 (CH3-3), 55.6 (8-OCH3).
- Fotso, S.; Mahmud, T.; Zabriskie, T.M.; Santosa, D.A.; Sulastri; Proteau, P.J. ngucyclinones from an Indonesian Streptomyces sp. J. Nat. Prod. 2008, 71, 61–65. [Google Scholar] [CrossRef]
- Sun, C.H.; Wang, Y.; Wang, Z.; Zhou, J.Q.; Jin, W.Z.; You, X.F.; Gao, H.; Zhao, L.X.; Si, S.Y.; Li, X. Chemomicin A, a new angucyclinone antibiotic produced by Nocardia mediterranei subsp. kanglensis 1747-64. J. Antibiot. 2007, 60, 211–215. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Maksimenka, K.; Hamm, A.; Gulder, T.A.M.; Dieter, A.; Bull, A.T.; Stach, J.E.M.; Kocher, N.; Müller, W.E.G.; et al. Gephyromycin, the first bridged angucyclinone, from Streptomyces griseus strain NTK 14. Phytochemistry 2005, 66, 1366–1373. [Google Scholar]
- Tsukuda, E.; Tanaka, T.; Ochiai, K.; Kondo, H.; Yoshida, M.; Agatsuma, T.; Saitoh, Y.; Teshiba, S.; Matsuda, Y. EI-1507-1 and -2, novel interleukin-1 beta converting enzyme inhibitors produced by Streptomyces sp. E-1507. J. Antibiot. 1996, 49, 333–339. [Google Scholar] [CrossRef]
- Carsten, P.; Axel, Z.; Beil, W. New biologically active rubiginones from Streptomyces sp. J. Antibiot. 2000, 53, 329–336. [Google Scholar] [CrossRef]
- Parker, K.A.; Ding, Q.J. A general approach to angucyclines: Synthesis of hatomarubigin A, rubiginone B2, antibiotic X-1488E, 6-hydroxytetrangulol, and tetrangulol. Tetrahedron 2000, 56, 10249–10254. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, H.P.; Cui, H.L.; Li, Z.G.; Xie, Z.P.; Pu, Y.; Li, F.C.; Qin, S. A new anthracene derivative from marine Streptomyces sp. W007 exhibiting highly and selectively cytotoxic activities. Mar. Drugs 2011, 9, 1502–1509. [Google Scholar] [CrossRef]
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xie, Z.; Liu, B.; Wang, H.; Yang, S.; Zhang, H.; Wang, Y.; Ji, N.; Qin, S.; Laatsch, H. Kiamycin, a Unique Cytotoxic Angucyclinone Derivative from a Marine Streptomyces sp. Mar. Drugs 2012, 10, 551-558. https://doi.org/10.3390/md10030551
Xie Z, Liu B, Wang H, Yang S, Zhang H, Wang Y, Ji N, Qin S, Laatsch H. Kiamycin, a Unique Cytotoxic Angucyclinone Derivative from a Marine Streptomyces sp. Marine Drugs. 2012; 10(3):551-558. https://doi.org/10.3390/md10030551
Chicago/Turabian StyleXie, Zeping, Bing Liu, Hongpeng Wang, Shengxiang Yang, Hongyu Zhang, Yipeng Wang, Naiyun Ji, Song Qin, and Hartmut Laatsch. 2012. "Kiamycin, a Unique Cytotoxic Angucyclinone Derivative from a Marine Streptomyces sp." Marine Drugs 10, no. 3: 551-558. https://doi.org/10.3390/md10030551






