Cyanobacterial Neurotoxin β-N-Methylamino-L-alanine (BMAA) in Shark Fins
Abstract
:1. Introduction
2. Results and Discussion
| Species | Scientific Name | Location | Month | Cyanobacterial Blooms | |
|---|---|---|---|---|---|
| Blacknose a | Carcharhinus acronotus | 25.62099°N | 80.15602°W | August | not present |
| Blacktip b | Carcharhinus limbatus | 25.00644°N | 80.99969°W | March | present |
| Blacktip b | Carcharhinus limbatus | 25.00644°N | 80.99969°W | September | present |
| Blacktip a | Carcharhinus limbatus | 25.59968°N | 80.15205°W | July | not present |
| Blacktip b | Carcharhinus limbatus | 25.01109°N | 80.99832°W | September | present |
| Blacktip b | Carcharhinus limbatus | 25.00644°N | 80.99969°W | March | present |
| Blacktip a | Carcharhinus limbatus | 25.62592°N | 80.15442°W | October | not present |
| Blacktip a | Carcharhinus limbatus | 25.61905°N | 80.1714°W | October | not present |
| Blacktip a | Carcharhinus limbatus | 25.64757°N | 80.1881°W | April | not present |
| Blacktip a | Carcharhinus limbatus | 25.67199°N | 80.18144°W | September | not present |
| Blacktip b | Carcharhinus limbatus | 25.01089°N | 81.00419°W | September | present |
| Blacktip b | Carcharhinus limbatus | 25.00976°N | 81.00079°W | September | present |
| Blacktip b | Carcharhinus limbatus | 25.01715°N | 81.01056°W | September | present |
| Bonnethead a | Sphyrna tiburo | 25.36711°N | 80.14806°W | March | not present |
| Bonnethead a | Sphyrna tiburo | 25.36711°N | 80.14806°W | March | not present |
| Bonnethead a | Sphyrna tiburo | 25.40807°N | 80.21806°W | October | not present |
| Bull b | Carcharhinus leucas | 25.01715°N | 81.01056°W | September | present |
| Bull b | Carcharhinus leucas | 25.01309°N | 81.00129°W | September | present |
| Great Hammerhead a | Sphyrna mokarran | 25.62138°N | 80.15656°W | July | not present |
| Great Hammerhead b | Sphyrna mokarran | 25.01715°N | 81.01056°W | September | present |
| Lemon b | Negaprion brevirostris | 25.00644°N | 80.99969°W | June | present |
| Lemon b | Negaprion brevirostris | 25.00644°N | 80.99969°W | June | present |
| Nurse a | Ginglymostoma cirratum | 25.61942°N | 80.1835°W | September | not present |
| Nurse b | Ginglymostoma cirratum | 24.88335°N | 80.84475°W | April | present |
| Nurse b | Ginglymostoma cirratum | 25.00644°N | 80.99969°W | March | present |
| Nurse a | Ginglymostoma cirratum | 25.62311°N | 80.15626°W | August | not present |
| Nurse a | Ginglymostoma cirratum | 25.60062°N | 80.15214°W | August | not present |
| Nurse a | Ginglymostoma cirratum | 25.60569°N | 80.1534°W | August | not present |
| Nurse a | Ginglymostoma cirratum | 25.62311°N | 80.15626°W | August | not present |
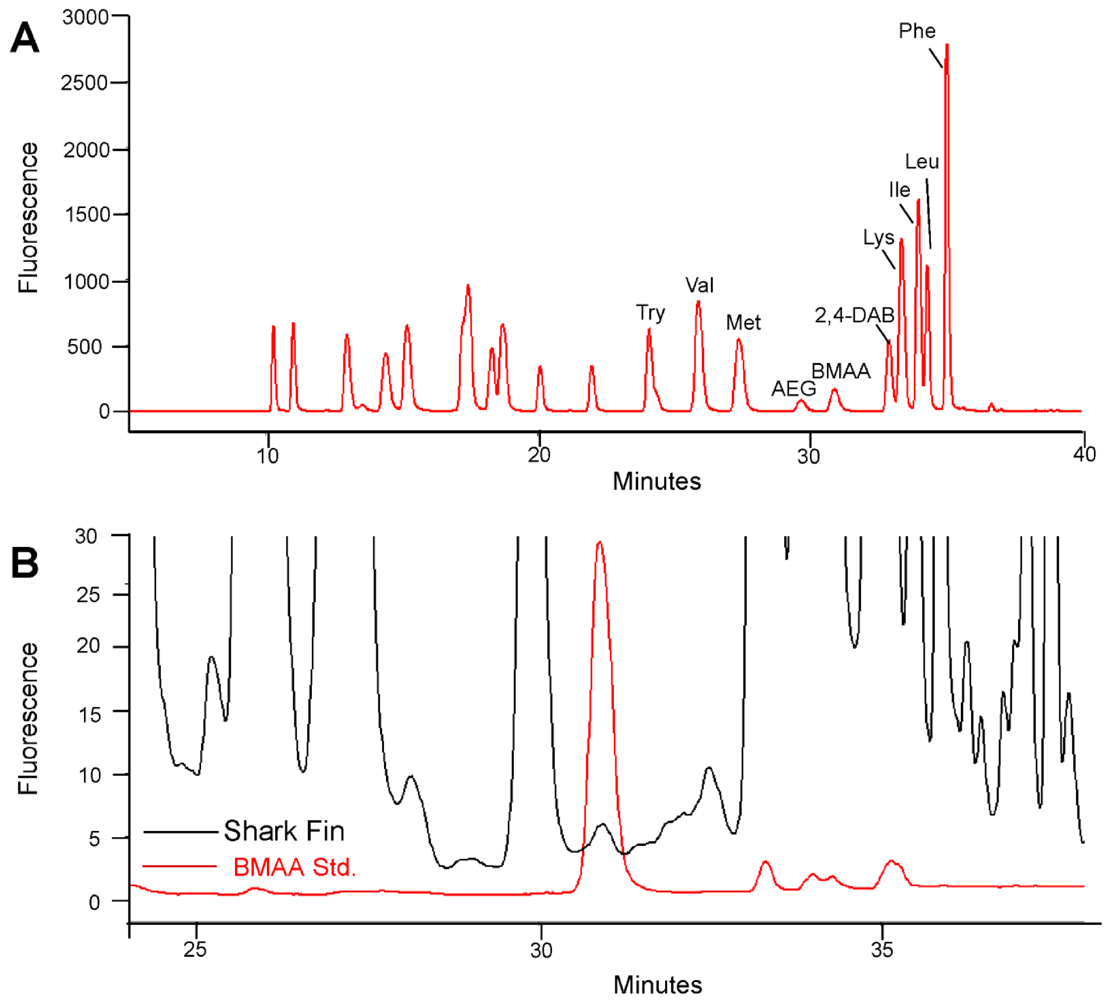
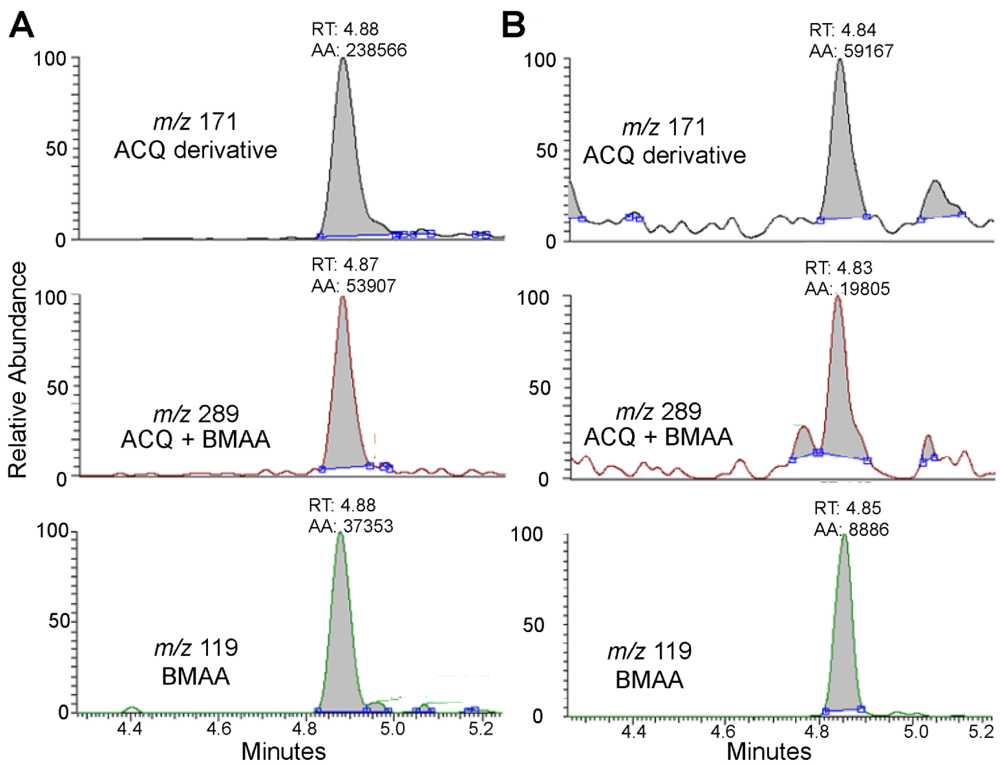
| Species | Size (cm) | BMAA Mean (ng/mg) | SE | BMAA (ng/100 cm shark) |
|---|---|---|---|---|
| Blacknose a (1) | 120 | 1,663 | 1,386 | |
| Blacktip b,* (4) | 61 | 280 | 84 | 460 |
| Blacktip b,* (4) | 99 | 144 | 18 | 210 |
| Blacktip a (1) | 162 | ND | ND | |
| Blacktip b,* (1) | 165 | ND | ND | |
| Blacktip b,* (1) | 173 | 286 | 165 | |
| Blacktip a (1) | 174 | 168 | 97 | |
| Blacktip a (1) | 177 | 247 | 140 | |
| Blacktip a (1) | 148 | 794 | 537 | |
| Blacktip a (1) | 155 | 811 | 522 | |
| Blacktip b,* (1) | 165 | 303 | 184 | |
| Blacktip b,* (1) | 165 | 745 | 453 | |
| Blacktip b,* (1) | 168 | 252 | 150 | |
| Bonnethead a (4) | 76 | 632 | 96 | 860 |
| Bonnethead a (4) | 79 | 320 | 59 | 408 |
| Bonnethead a (4) | 77 | 1,836 | 364 | 2,385 |
| Bull b,* (4) | 163 | 232 | 60 | 142 |
| Bull b,* (4) | 183 | 264 | 96 | 144 |
| Great Hammerhead a (4) | 247 | 1,528 | 212 | 619 |
| Great Hammerhead b,* (4) | 175 | 528 | 211 | 291 |
| Lemon b,* (4) | 168 | 556 | 210 | 332 |
| Lemon b,* (4) | 201 | 628 | 66 | 312 |
| Nurse a (1) | 226 | 223 | 99 | |
| Nurse b,* (1) | 213 | 169 | 79 | |
| Nurse b,* (1) | 168 | 161 | 96 | |
| Nurse a (1) | 165 | ND | ND | |
| Nurse a (1) | 235 | ND | ND | |
| Nurse a (1) | 207 | ND | ND | |
| Nurse a (1) | 241 | ND | ND |
| Organ | BMAA Mean (ng/mg) | SE | BMAA (ng/100 cm of shark) |
|---|---|---|---|
| Kidney (3) | 1450 | 687 | 598 |
| Liver (4) | 588 | 81 | 243 |
| Fin (8) | 1028 | 211 | 487 |
| Muscle (3) | 58 | 41 | 24 |
| Heart (2) | ND | ND |
3. Experimental Section
3.1. Sample Collection
3.2. Fluorescence HPLC Methods for Analysis of Protein-Associated BMAA
3.3. Triple Quadrupole LC/MS/MS
4. Conclusions
Acknowledgments
References
- Feretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet earth. Science 2011, 333, 301–306. [Google Scholar]
- Baum, J.K.; Myers, R.A.; Kehler, D.G.; Worm, B.; Harley, S.J.; Doherty, P.A. Collapse and conservation of shark populations in the northwest Atlantic. Science 2003, 299, 389–392. [Google Scholar]
- Dulvy, N.K.; Baum, J.K.; Clarke, S. You can swim but you can’t hide the global status and conservation of oceanic pelagic sharks. Aquat. Conserv. 2008, 18, 459–482. [Google Scholar]
- Lucifora, L.O.; Garcia, V.B.; Worm, B. Global diversity hotspots and conservation priorities for sharks. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Clarke, S.; McAllister, M.K.; Milner-Gulland, E.J.; Kirkwood, G.P.; Mechielsens, C.G.J.; Agnew, D.J.; Pikitch, E.K.; Nakano, H.; Shivji, M.S. Global estimates of shark catches using trade records from commercial markets. Ecol. Lett. 2006, 9, 1115–1126. [Google Scholar]
- Verlecar, X.N.; Snigdha, D.S.R.; Dhargalkar, V.K. Shark hunting—An indiscriminate trade endangering elasmobranchs to extinction. Curr. Sci. 2007, 92, 1078–1082. [Google Scholar]
- Hueter, R.E.; Fong, W.G.; Henderson, G.; French, M.F.; Manire, C.A. Methylmercury concentration in shark muscle by species, size and distribution of sharks in Florida coastal waters. Water Air Soil Pollut. 1995, 80, 893–899. [Google Scholar]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.G.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C.; Sacks, O.W. Occurrence of β-methylamino-L-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004, 110, 267–269. [Google Scholar]
- Anderson, D.M.; Gilbert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar]
- Jonasson, S.; Eriksson, J.; Berntzon, L.; Spacil, Z.; Ilag, L.L.; Ronnevi, L.; Rasmussen, U.; Bergman, B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 9252–9257. [Google Scholar]
- Li, A.; Tian, Z.; Li, J.; Yu, R.; Banack, S.A.; Wang, Z. Detection of the neurotoxin BMAA within cyanobacteria isolated from freshwater in China. Toxicon 2010, 55, 947–953. [Google Scholar]
- Faassen, E.J.; Gillissen, F.; Zweers, H.A.J.; Lurling, M. Determination of the neurotoxins BMAA (β-N-methylamino-L-alanine) and DAB (α-,γ-diaminobutyric acid) by LC-MS/MS in Dutch urban waters with cyanobacterial blooms. Amyotroph. Lateral Scler. 2009, 2, 79–84. [Google Scholar]
- Esterhuizen, M.; Downing, T.G. Beta-N-methylamino-L-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol. Environ. Saf. 2008, 71, 309–313. [Google Scholar]
- Metcalf, J.S.; Banack, S.A.; Lindsay, J.; Morrison, L.F.; Cox, P.A.; Codd, G.A. Co-occurrence of β-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British water bodies, 1990–2004. Environ. Microbiol. 2008, 10, 702–708. [Google Scholar]
- Johnson, H.E.; King, S.R.; Banack, S.A.; Webster, C.; Callanaupa, W.J.; Cox, P.A. Cyanobacteria (Nostoc commune) used as a dietary item in the Peruvian highlands produce the neurotoxic amino acid BMAA. J. Ethnopharmacol. 2008, 118, 159–165. [Google Scholar]
- Banack, S.A.; Johnson, H.E.; Cheng, R.; Cox, P.A. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar. Drugs 2007, 5, 180–196. [Google Scholar]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial blooms and the occurrence of the neurotoxin, beta-N-methylamino-L-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae 2010, 9, 620–635. [Google Scholar]
- Cohen, S.A.; Michaud, D.P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar]
- Banack, S.A.; Murch, S.J.; Cox, P.A. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J. Ethnopharmacol. 2006, 106, 97–104. [Google Scholar]
- Murch, S.J.; Cox, P.A.; Banack, S.A. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc. Natl. Acad. Sci. USA 2004, 101, 12228–12231. [Google Scholar]
- Hammerschlag, N.; Gallagher, A.J.; Lazarre, D.M.; Slonim, C. Range extension of endangered great hammerhead shark Sphyrna mokarran in the Northwest Atlantic: Preliminary data and significance for conservation. Endanger. Species Res. 2011, 13, 111–116. [Google Scholar]
- Field, N.C.; Caller, T.A.; Stommel, E.W. An explanation for the changes in collagen in sporadic Amyotrophic Lateral Sclerosis. Med. Hypotheses 2011, 77, 565–567. [Google Scholar]
- Banack, S.A.; Cox, P.A. Biomagnification of cycad neurotoxins in flying foxes: Implications for ALS-PDC in Guam. Neurology 2003, 6, 387–389. [Google Scholar]
- IUCN Red List of Threatened Species. Version 2011.1. Available online: www.iucnredlist.org (accessed on 15 February 2012).
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mondo, K.; Hammerschlag, N.; Basile, M.; Pablo, J.; Banack, S.A.; Mash, D.C. Cyanobacterial Neurotoxin β-N-Methylamino-L-alanine (BMAA) in Shark Fins. Mar. Drugs 2012, 10, 509-520. https://doi.org/10.3390/md10020509
Mondo K, Hammerschlag N, Basile M, Pablo J, Banack SA, Mash DC. Cyanobacterial Neurotoxin β-N-Methylamino-L-alanine (BMAA) in Shark Fins. Marine Drugs. 2012; 10(2):509-520. https://doi.org/10.3390/md10020509
Chicago/Turabian StyleMondo, Kiyo, Neil Hammerschlag, Margaret Basile, John Pablo, Sandra A. Banack, and Deborah C. Mash. 2012. "Cyanobacterial Neurotoxin β-N-Methylamino-L-alanine (BMAA) in Shark Fins" Marine Drugs 10, no. 2: 509-520. https://doi.org/10.3390/md10020509
APA StyleMondo, K., Hammerschlag, N., Basile, M., Pablo, J., Banack, S. A., & Mash, D. C. (2012). Cyanobacterial Neurotoxin β-N-Methylamino-L-alanine (BMAA) in Shark Fins. Marine Drugs, 10(2), 509-520. https://doi.org/10.3390/md10020509




