Cytotoxic Activity of Semi-Synthetic Derivatives of Elatol and Isoobtusol
Abstract
:1. Introduction

2. Results and Discussion
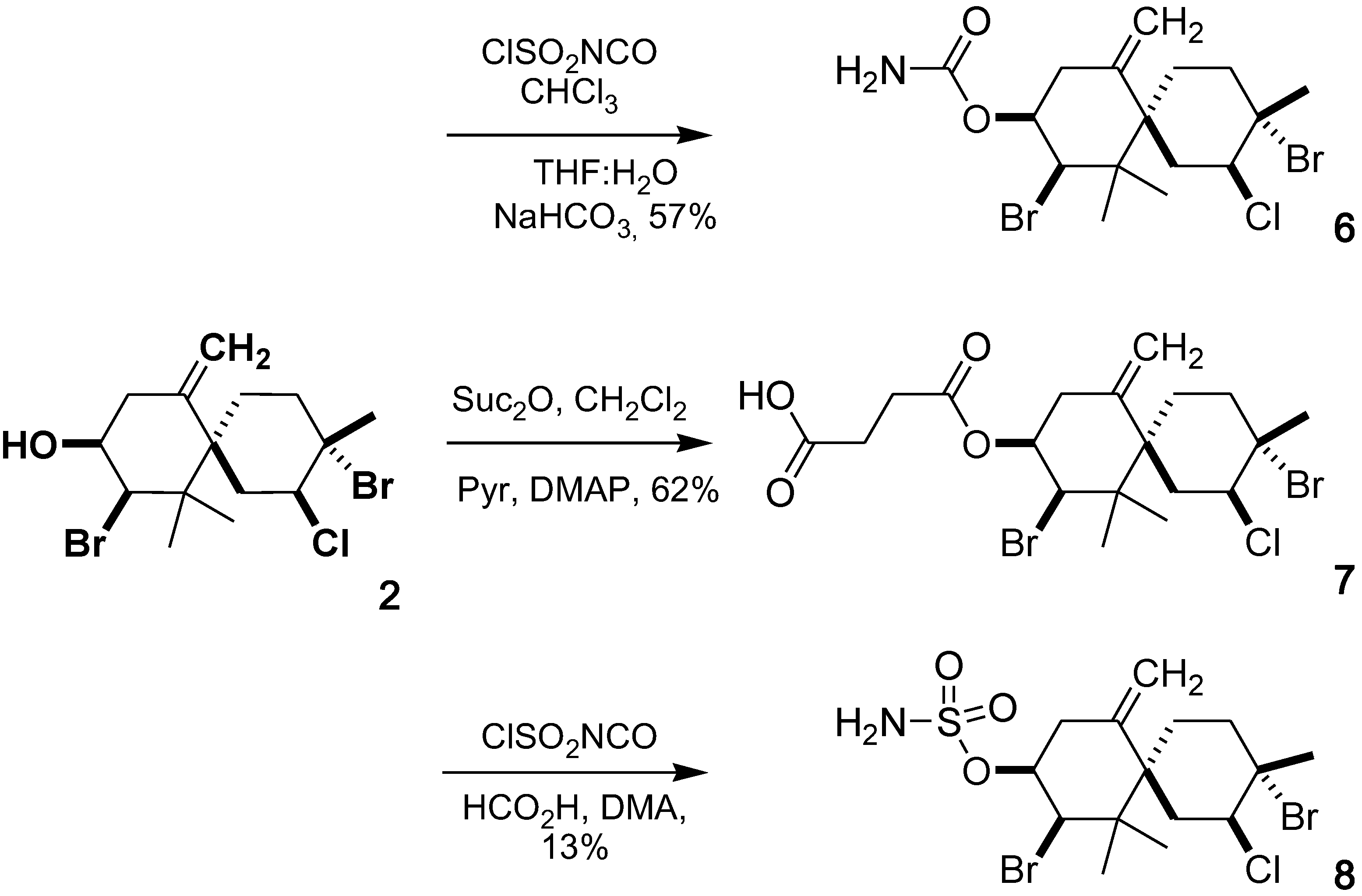
2.1. Chemistry

2.2. Cytotoxic Activity
| Compound | Cell lines [CC50a (μM)] | |
|---|---|---|
| A549 | RD | |
| 1 | 7.56 ± 0.19 | 11.22 ± 1.63 |
| 2 | 14.24 ± 3.43 | 6.24 ± 1.11 |
| 3 | 21.93 ± 1.27 | 13.26 ± 0.76 |
| 4 | 39.05 ± 4.81 | 21.23 ± 7.16 |
| 5 | >100 | 41.53 ± 0.36 |
| 6 | 39.57 ± 2.07 | 23.83 ± 5.28 |
| 7 | 10.74 ± 2.52 | 4.93 ± 0.52 |
| 8 | 23.85 ± 5.21 | 20.48 ± 1.44 |
| Paclitaxel | 0.260 ± 0.027 | 0.025 ± 0.004 |
3. Experimental
3.1. General
3.2. Algal Material
3.3. Synthesis
3.3.1. Elatol 9-Carbamate (3)
3.3.2. Elatol 9-Hemisuccinate (4)
3.3.3. Elatol 9-Sulfamate (5)
3.3.4. Isoobtusol 9-Carbamate (6)
3.3.5. Isoobtusol 9-Hemisuccinate (7)
3.3.6. Isoobtusol 9-Sulfamate (8)
3.4. Cell Lines
3.5. MTT Assay
3.6. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Mayer, A.M.; Rodríguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine nature products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef]
- Sims, J.J.; Lin, G.H.Y.; Wing, R.M. Marine natural products: Elatol, a halogenated sesquiterpene alcohol from the red alga Laurencia elata. Tetrahedron Lett. 1974, 39, 3487–3490. [Google Scholar]
- König, G.M.; Wright, A.D. Sesquiterpene content of the antibacterial dichlormethane extract of the red alga Laurencia obtusa. Planta Med. 1997, 63, 186–187. [Google Scholar] [CrossRef]
- Juagdan, E.G.; Kalidindi, R.; Scheuer, P. Two new chamigranes from an hawaiian red alga, Laurencia cartikzginea. Tetrahedron 1997, 2, 521–528. [Google Scholar]
- Vairappan, C.S. Potent antibacterial activity of halogenated metabolites from Malaysian red algae, Laurencia majuscula (Rhodomelaceae, Ceramiales). Biomol. Eng. 2003, 20, 255–259. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Anangdan, S.P.; Tan, K.L.; Matsunaga, S. Role of secondary metabolites as defense chemicals against ice-ice disease bacteria in biofouler at carrageenophyte farms. J. Appl. Phycol. 2009, 22, 305–311. [Google Scholar]
- Lhullier, C.; Donnangelo, A.; Caro, M.; Palermo, J.A.; Horta, P.A.; Falkenberg, M.; Schenkel, E.P. Isolation of elatol from Laurencia microcladia and its palatability to the sea urchin Echinometra lucunter. Biochem. Syst. Ecol. 2009, 37, 254–259. [Google Scholar] [CrossRef]
- Granado, I.; Caballero, P. Chemical defense in the seaweed Laurencia obtusa (Hudson) Lamouroux. Sci. Mar. 1995, 59, 31–39. [Google Scholar]
- De Nys, R.; Leya, T.; Maximilien, R.; Afsar, A.; Nair, P.S.R.; Steinberg, P.D. The need for standardised broad scale bioassay testing: A case study using the red alga Laurencia rigida. Biofouling 1996, 10, 213–224. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Daitoh, M.; Suzuki, M.; Abe, T.; Masuda, M. Antibacterial halogenated metabolites from the Malaysian Laurencia species. Phytochemistry 2001, 58, 291–297. [Google Scholar]
- König, G.M.; Wright, A.D. Laurencia rigida: Chemical investigations of its antifouling dichloromethane extract. J. Nat. Prod. 1997, 60, 967–970. [Google Scholar] [CrossRef]
- Santos, A.O.; Veiga-Santos, P.; Ueda-Nakamura, T.; Dias-Filho, B.T.; Sudatti, D.B.; Bianco, E.M.; Nakamura, C.V. Effect of Elatol, Isolated from Red Seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Pelizzaro-Rocha, K.J.; Santos, A.O.; Ueda-Nakamura, T.; Dias-Filho, B.T.; Silva, S.O.; Sudatti, D.B.; Bianco, E.M.; Nakamura, C.V. In vitro anti-trypanosomal activity of elatol isolated from red seaweed Laurencia dendroidea. Parasitology 2010, 137, 1661–1670. [Google Scholar] [CrossRef]
- Dias, T.; Brito, I.; Moujir, L.; Paiz, N.; Darias, J.; Cueto, M. Cytotoxic Sesquiterpenes from Aplysia dactylomela. J. Nat. Prod. 2005, 68, 1677–1679. [Google Scholar] [CrossRef]
- Campos, A.; Souza, C.B.; Lhullier, C.; Schenkel, E.P.; Ribeiro-do-Valle, R.M.; Siqueira, J.M. Antitumoral effects of elatol, a marine derivative compound obtained from red algae Laurencia microcladia. J. Pharm. Pharmacol. 2012, 64, 1146–1154. [Google Scholar]
- González, A.G.; Darias, J.; Díaz, A.; Fourneron, J.D.; Martín, J.D.; Pérez, C. Evidence for the biogenesis of halogenated chamigrenes from the red alga Laurencia obtusa. Tetrahedron Lett. 1976, 17, 3051–3054. [Google Scholar] [CrossRef]
- Djuric, Z.; Heilbrun, L.K.; Lababidi, S.; Everett-Bauer, C.K.; Fariss, M.W. Growth inhibition of MCF-7 and MCF-10A human breast cells by α-tocopheryl hemisuccinate, cholesteryl hemisuccinate and their ether analogs. Cancer Lett. 1997, 111, 133–139. [Google Scholar] [CrossRef]
- Kogure, K.; Manabe, S.; Hama, S.; Tokumura, A.; Fukuzawa, K. Potentiation of anti-cancer effect by intravenous administration of vesiculated α-tocopheryl hemisuccinate on mouse melanoma in vivo. Cancer Lett. 2003, 192, 19–24. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Janout, V.; Zhang, L.; Regen, S.L. Ion conductors derived from cholic acid and spermine: Importance of facial hydrophilicity on Na+ transport and membrane selectivity. J. Am. Chem. Soc. 2001, 123, 7691–7696. [Google Scholar] [CrossRef]
- Duran, F.J.; Ghini, A.A.; Dauban, P.; Dodd, R.H.; Burton, G. Synthesis of 6,19-sulfamidate bridged pregnanes. J. Org. Chem. 2005, 70, 8613–8616. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lang, K.L.; Silva, I.T.; Zimmermann, L.A.; Lhullier, C.; Mañalich Arana, M.V.; Palermo, J.A.; Falkenberg, M.; Simões, C.M.O.; Schenkel, E.P.; Durán, F.J. Cytotoxic Activity of Semi-Synthetic Derivatives of Elatol and Isoobtusol. Mar. Drugs 2012, 10, 2254-2264. https://doi.org/10.3390/md10102254
Lang KL, Silva IT, Zimmermann LA, Lhullier C, Mañalich Arana MV, Palermo JA, Falkenberg M, Simões CMO, Schenkel EP, Durán FJ. Cytotoxic Activity of Semi-Synthetic Derivatives of Elatol and Isoobtusol. Marine Drugs. 2012; 10(10):2254-2264. https://doi.org/10.3390/md10102254
Chicago/Turabian StyleLang, Karen L., Izabella T. Silva, Lara A. Zimmermann, Cíntia Lhullier, Maria V. Mañalich Arana, Jorge A. Palermo, Miriam Falkenberg, Cláudia M. O. Simões, Eloir P. Schenkel, and Fernando J. Durán. 2012. "Cytotoxic Activity of Semi-Synthetic Derivatives of Elatol and Isoobtusol" Marine Drugs 10, no. 10: 2254-2264. https://doi.org/10.3390/md10102254
APA StyleLang, K. L., Silva, I. T., Zimmermann, L. A., Lhullier, C., Mañalich Arana, M. V., Palermo, J. A., Falkenberg, M., Simões, C. M. O., Schenkel, E. P., & Durán, F. J. (2012). Cytotoxic Activity of Semi-Synthetic Derivatives of Elatol and Isoobtusol. Marine Drugs, 10(10), 2254-2264. https://doi.org/10.3390/md10102254




