Long-Chain Acetylenic Ketones from the Micronesian Sponge Haliclona sp. Importance of the 1-yn-3-ol Group for Antitumor Activity
Abstract
:Introduction
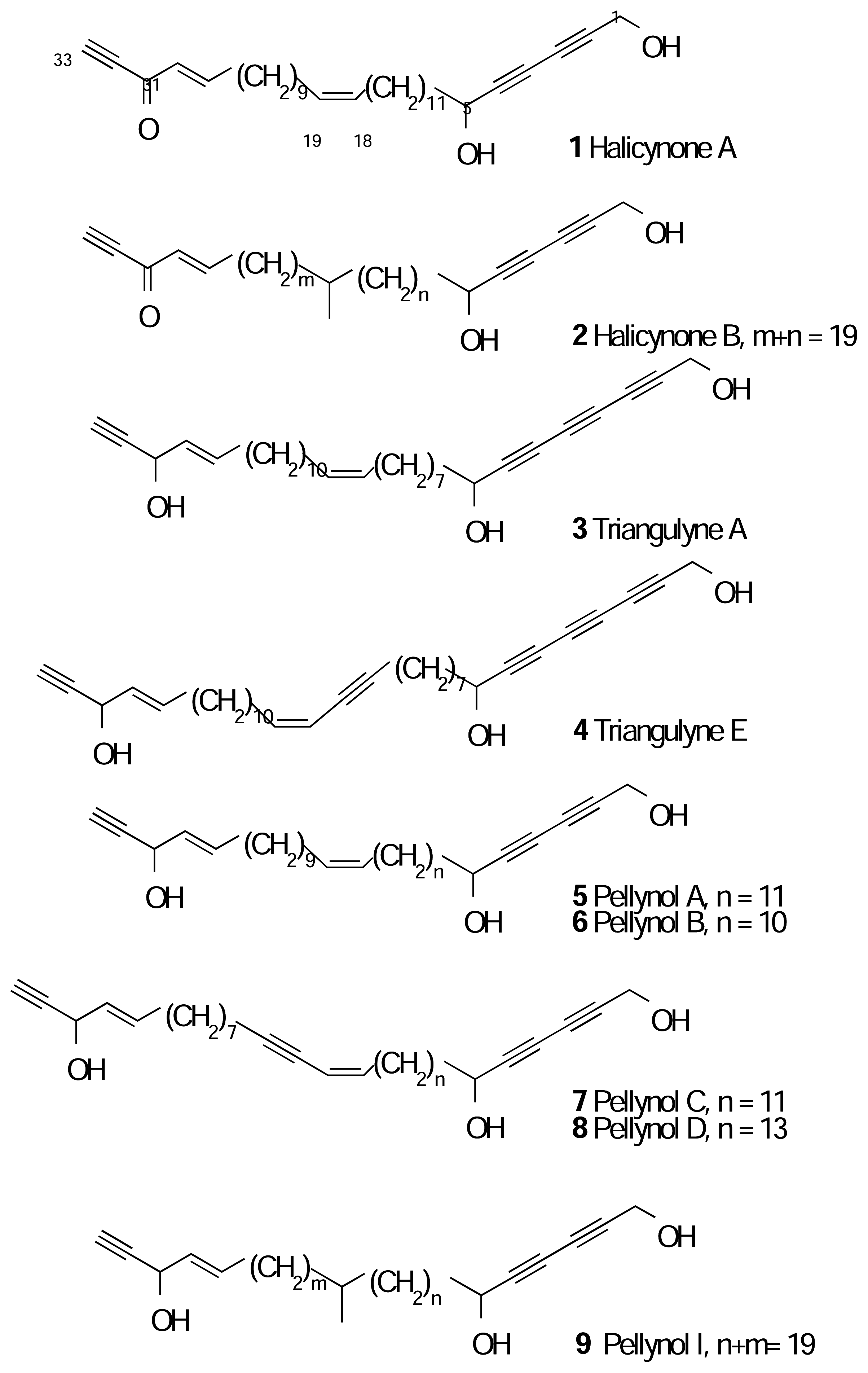
Results and Discussion
Conclusions
Experimental
General
Spectral Data
Oxidation of Pellynol A (5) with MnO2: Conversion to 1
Acknowledgements
- Sample Availability: Samples are available from the authors.
References and Notes
- For examples, see Quinoa, E.; Crews, P. Melynes, polyacetylene constituents from a Vanuatu marine sponge. Tetrahedron Lett 1988, 29(17), 2037–2040. [Google Scholar]; Youssef, D. T. A.; Yoshida, W.Y.; Kelly, M.; Scheuer, P. J. New cytotoxic polyacetylenes from a Red Sea sponge Callyspongia species. J. Nat. Prod 2000, 63, 1406–1410. [Google Scholar]; Guo, Y.; Gavagnin, M.; Trivellone, E.; Cimino, G. Further structural studies on the Petroformynes. J. Nat. Prod 1995, 58(5), 712–722. [Google Scholar]
- Kim, D.-K.; Lee, M.-Y.; Lee, H. S.; Lee, D. S.; Lee, J.-R.; Lee, B.-J.; Jung, J. H. Polyacetylenes from a marine sponge Petrosia sp. inhibit DNA replication at the level of initiation. Cancer Lett 2002, 185(1), 95–101. [Google Scholar]
- Kobayashi, M.; Kawazoe, K.; Okamoto, T.; Sasaki, T.; Kitagawa, I. Chem. Pharm. Bull 1994, 42, 19.
- For example, pellynols A–D: Fu, X.; Abbas, S. A.; Schmitz, F. J.; Vidavsky, I.; Gross, M. L.; Laney, M.; Schatzmann, R. C.; Cabuslay, R. D. New acetylenic metabolites from the marine sponge Pellina triangulata. Tetrahedron 1997, 53, 799–814. [Google Scholar]; Pellynols E–H were isolated from Theonella sp.; Fu, X.; Schmitz, F. J.; Kelly, M. Swinholides and new acetylenic compounds from an undescribed species of Theonella Sponge. J. Nat. Prod 1999, 62, 1336–1338. [Google Scholar], while pellynol I was reported from a South African Pellina sp.; Rashid, M.; Gustafson, K. R.; Boyd, M. R. Pellynol I, a new cytotoxic polyacetylene from the sponge Pellina sp. Nat. Prod. Lett 2000, 14(5), 287–392. [Google Scholar]
- Dai, J–R.; Hallock, Y. F.; Cardellina, J. H., III; Gray, G. N.; Boyd, M. R. Triangulynes A–H and triangulynic acid, new cytotoxic polyacetylenes from the marine sponge Pellina triangulate. J. Nat. Prod 1996, 59, 860–866. [Google Scholar]
- Nomenclature considerations define the primary alcohol in pellynol A (5) at C1 and the terminal acetylene carbon at C33. Systematic numbering of the corresponding ketones 1 and 2 would require reversed ordering of locant numbers. In order to emphasis the importance of the terminal propargylic alcohol and retain a useful reference point, we use the terms ‘1-yn-3-ol’ and ‘1-yn-3-one’ to refer to functionality numbered from the ω-terminus with respect to pellynol numbering.
- Compounds 23 and 4 were not tested against HCT-116 due to insufficient material or decomposition.
- None of the compounds showed significant antifungal activity (Candida glabrata) in their pure states.
- This is surprising as other compounds in this class, such as 3–9 exhibit cytotoxicity to mammalian cells.
- Aoki, S.; Matsui, K.; Tanaka, K.; Satari, R.; Kobayashi, M. Lembehyne A, a novel neuritogenic polyacetylene, from a marine sponge of Haliclona sp. Tetrahedron 2002, 56(51), 9945–9948. [Google Scholar]Aoki, S.; Matsui, K.; Wei, H.; Murakami, N.; Kobayashi, M. Structure-activity relationship of neuritogenic spongean acetylene alcohols, lembehynes. Tetrahedron 2002, 58(27), 5417–5422. [Google Scholar]Aoki, S.; Matsui, K.; Takata, T.; Hong, W.; Kobayashi, M. Lembehyne A, a spongean polyacetylene, induces neuronal differentiation in neuroblastoma cell. Biochem. Biophys. Res. Commun 2001, 289(2), 558–563. [Google Scholar]Aoki, S.; Matsui, K.; Tanaka, K.; Satari, R.; Kobayashi, M. Lembehyne A, a novel neuritogenic polyacetylene, from a marine sponge of Haliclona sp. Tetrahedron 2000, 56(51), 9945–9948. [Google Scholar]
- Recent photoaffinity labeling experiments have identified a 30 kDa protein from Neuro–2A cells as the target of the natural product lembehyne A. Aoki, S.; Matsui, K.; Takata, T.; Kobayashi, M. In situ photoaffinity labeling of the target protein for lembehyne A, a neuronal differentiation inducer. FEBS Lett 2003, 544(1–3), 223–7. [Google Scholar]
- Searle, P. A.; Molinski, T. F. Five new alkaloids from the tropical ascidian, Lissoclinum sp. Lissoclinotoxin A is chiral. J. Org. Chem 1994, 59, 6600–6605. [Google Scholar]
- Jones, R. N.; Barry, A. L.; Gavan, T. L.; Washington, J.A., III. Manual of Clinical Microbiology, 4 th. ed; Lennette, A., Balows, A., Hausler, W. J., Shadomy, H. J., Eds.; American Society of Microbiology: Washington, D.C, 1985. [Google Scholar]
- MTS: (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium, inner salt), Promega CellTiter 96® Aqueous cell proliferation assay, Technical Bulletin No. 169.
- Compounds were assayed with compounds in DMSO (final concentration, 1% v/v) and run against etoposide as positive control. HCT-116 cells were incubated in 96-well plates for 72 h before addition of MTS. Well absorbances (λ 490 nm) were corrected for background and expressed as a percentage of the negative control (DMSO, only).
© 2003 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhou, G.-X.; Molinski, T.F. Long-Chain Acetylenic Ketones from the Micronesian Sponge Haliclona sp. Importance of the 1-yn-3-ol Group for Antitumor Activity. Mar. Drugs 2003, 1, 46-53. https://doi.org/10.3390/md101046
Zhou G-X, Molinski TF. Long-Chain Acetylenic Ketones from the Micronesian Sponge Haliclona sp. Importance of the 1-yn-3-ol Group for Antitumor Activity. Marine Drugs. 2003; 1(1):46-53. https://doi.org/10.3390/md101046
Chicago/Turabian StyleZhou, Guang-Xiong, and Tadeusz F. Molinski. 2003. "Long-Chain Acetylenic Ketones from the Micronesian Sponge Haliclona sp. Importance of the 1-yn-3-ol Group for Antitumor Activity" Marine Drugs 1, no. 1: 46-53. https://doi.org/10.3390/md101046



